DETERMINATION OF THE ENTROPY CHANGE FOR ELECTRODE REACTION AND DILUTE ENTHALPY OF SOME IONS BY THERMO-ELETROCHEMICAL TECHNOLOGY
来源期刊:中南大学学报(英文版)1998年第1期
论文作者:Fang Zheng Guo Lin Zhang Hengzhong Zhang Pingming
文章页码:39 - 41
Key words:thermo-electrochemistry; entropy; dilute enthalpy
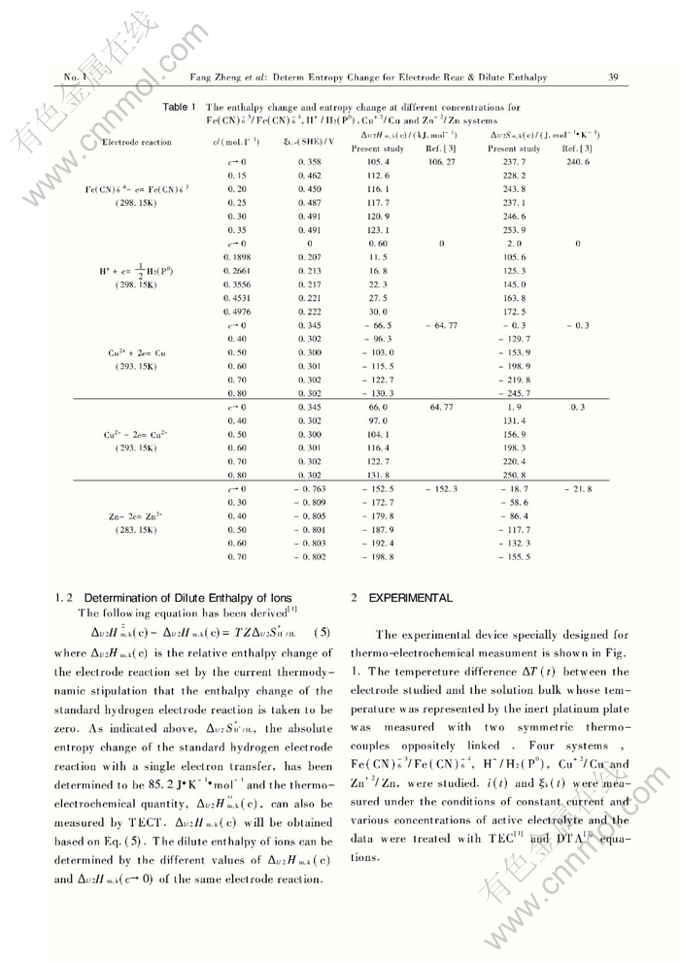
Abstract: Based on thermo-electrochemical equation for an electrode reaction, the entropy change of it can be obtainedby the thermo -electrochemical technology ( TECT ) . The entropy changes of Fe ( CN )-36/ Fe ( CN )-46, H+/H2(P0), Cu+2/Cu and Zn+2/Zn electrode reaction systems and the dilute enthalpies of the H+, Cu2+ and Zn2+ ions under the ion concentrations studied have been determined by a specially designed thermo-electrochemical equipment. The enthalpy change and entropy change for the five systems at unlimitedly diluted concentrations agree well with the literature.