Effect of Ti on microstructure, mechanical properties and corrosion resistance of Zr-Ta-Ti alloys processed by spark plasma sintering
来源期刊:中南大学学报(英文版)2020年第8期
论文作者:杨海林 苗惊雷 薛国林 邢海霞 叶传仁 刘珏 阮建明
文章页码:2185 - 2197
Key words:spark plasma sintering; mechanical properties; microstructure; corrosion resistance; Zr alloy
Abstract: The microstructure, mechanical properties and corrosion resistance of Zr-30%Ta and Zr-25%Ta-5%Ti alloy prepared by spark plasma sintering (SPS) technology were investigated. The experimental results showed that the Zr-Ta-Ti alloys made by the SPS processing have a low level of porosity with the relative density of 96%-98%. The analyses of XRD and TEM revealed that the Zr-30Ta alloy consists of α+β phase, and the Zr-25Ta-5Ti alloy belongs to the near β type alloy containing a small amount of α and ω phases. With the addition of Ti, the elastic modulus of the alloys was decreased from (99.5±7.2) GPa for Zr-30Ta alloy to (73.6±6.3) GPa for Zr-25Ta-5Ti alloy. Furthermore, it is shown that, in comparison to CP-Ti and Ti-6Al-4V alloy, the Zr-Ta-Ti alloy produced in this work offers an improved corrosion resistance due to the more stable ZrO2 and Ta2O5 generated in the passivation film on the surface of the alloys. This study demonstrates that Zr-Ta-Ti alloys are a promising candidate of novel metallic biomaterials.
Cite this article as: XUE Guo-lin, YANG Hai-lin, XING Hai-xia, YE Chuan-ren, LIU Jue, MIAO Jing-lei, RUAN Jian-ming. Effect of Ti on microstructure, mechanical properties and corrosion resistance of Zr-Ta-Ti alloys processed by spark plasma sintering [J]. Journal of Central South University, 2020, 27(8): 2185-2197. DOI: https://doi.org/10.1007/s11771-020-4440-9.

J. Cent. South Univ. (2020) 27: 2185-2197
DOI: https://doi.org/10.1007/s11771-020-4440-9

XUE Guo-lin(薛国林)1, YANG Hai-lin(杨海林)1, XING Hai-xia(邢海霞)2, YE Chuan-ren(叶传仁)1,
LIU Jue(刘珏)3, MIAO Jing-lei(苗惊雷)4, RUAN Jian-ming(阮建明)1
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. Department of General Dentistry, Peking University, School and Hospital of Stomatology, Beijing 100081, China;
3. Hunan Province Key Laboratory of Engineering Rheology, Central South University of Forestry and Technology, Changsha 410004, China;
4. Department of Orthopedics, The Third Xiangya Hospital of Central South University,Changsha 410013, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The microstructure, mechanical properties and corrosion resistance of Zr-30%Ta and Zr-25%Ta-5%Ti alloy prepared by spark plasma sintering (SPS) technology were investigated. The experimental results showed that the Zr-Ta-Ti alloys made by the SPS processing have a low level of porosity with the relative density of 96%-98%. The analyses of XRD and TEM revealed that the Zr-30Ta alloy consists of α+β phase, and the Zr-25Ta-5Ti alloy belongs to the near β type alloy containing a small amount of α and ω phases. With the addition of Ti, the elastic modulus of the alloys was decreased from (99.5±7.2) GPa for Zr-30Ta alloy to (73.6±6.3) GPa for Zr-25Ta-5Ti alloy. Furthermore, it is shown that, in comparison to CP-Ti and Ti-6Al-4V alloy, the Zr-Ta-Ti alloy produced in this work offers an improved corrosion resistance due to the more stable ZrO2 and Ta2O5 generated in the passivation film on the surface of the alloys. This study demonstrates that Zr-Ta-Ti alloys are a promising candidate of novel metallic biomaterials.
Key words: spark plasma sintering; mechanical properties; microstructure; corrosion resistance; Zr alloy
Cite this article as: XUE Guo-lin, YANG Hai-lin, XING Hai-xia, YE Chuan-ren, LIU Jue, MIAO Jing-lei, RUAN Jian-ming. Effect of Ti on microstructure, mechanical properties and corrosion resistance of Zr-Ta-Ti alloys processed by spark plasma sintering [J]. Journal of Central South University, 2020, 27(8): 2185-2197. DOI: https://doi.org/10.1007/s11771-020-4440-9.
1 Introduction
Biomedical implant materials have attracted increasing attentions in the past decades. In general, the medical implants are mainly categorized as polymers, ceramics and metals, of which the metallic materials are more favorable as biomedical implant materials due to their excellent combination of strength and toughness in association with corrosion resistance [1, 2]. The conventional implant materials based on titanium and titanium alloys such as Ti-6Al-4V have been widely used. However, there are still many problems with these implant materials. Firstly, the elastic modulus of titanium and titanium alloys is at a level of 100-120 GPa, which is much higher than that of human bone forming stress shielding during implantation, resulting in osteoporosis and failure of implant. Secondly, the release of toxic ions may cause some health problems. It is revealed that aluminum and vanadium ions can cause allergic reactions when they enter the body fluids during implantation [3, 4].
Zr has similar chemical properties with Ti, and has also an excellent corrosion resistance [5,6]. In addition, it is indicated that Zr alloys performed a higher contact degree of bone-implant interface than Ti alloys [7]. Therefore, Zr and Zr alloys are considered the good potential candidates for implant materials. In the past decade, a variety of Zr-based alloys have been designed and investigated for the implants, such as Zr-xNb alloys [8], Zr–xTi alloys [9], Zr-2.5Sn alloy [10] and Zr–xMo [11] alloys. It is generally known that the elastic modulus of β-Zr(BCC) is much lower than that of the α-Zr(HCP). Therefore, it is necessary to make the Zr alloys contain more β-Zr phase for the implants application [12]. Ta element plays an important role in reducing the elastic modulus of Zr alloys, and it belongs to β phase stable element due to more β phase being retained at room temperature [13]. Ta also shows better corrosion resistance than Zr and Ti. In the body fluid environment, a passivation film of Ta2O5 is formed on the alloy surface, which is more stable than ZrO2 and TiO2 [14]. In addition, BALLA et al [15] found that Ta, having improved biocompatibility, is more likely to promote cell proliferation and differentiation than Ti. Some studies have found that Ta can reduce the loss of matrix metal in body fluids [16]. The previous studies also showed that Zr-Ta alloys exhibited low elastic modulus, good corrosion resistance and excellent biocompatibility [17]. It is revealed that Ti reduces the elastic modulus of Zr alloys and Ta alloys, and improves their corrosion resistance. However, there are few studies focusing on the effect of Ti addition on Zr-Ta alloy [3].
Compared with the alloys prepared by the conventional casting method, the refractory metals prepared by powder metallurgy (P/M) method have the advantages of low impurity content, fine grain size, and few subsequent thermomechanical processing [16-19]. Belonging to a new type of powder metallurgy technology, the spark plasma sintering (SPS) technology generates high- temperature plasma in the early stage of the sintering process, which will cause sintering promotion, achieving a rapid high-temperature field to realize rapid densification of powder particles. This technology has a variety of advantages such as rapid temperature increase, uniform grain size and high density for the resultant materials, short sintering time, and low sintering temperature [20-22]. In this study, the Zr-Ta-Ti alloy was prepared by the SPS technology, being demonstrated to be a potential dental metal material with good comprehensive performance. The effect of Ti addition on the microstructure, mechanical properties and corrosion resistance of the Zr-Ta-Ti alloy was investigated.
2 Experimental procedure
2.1 Materials preparation
In this work, pure zirconium powder, pure titanium powder, and pure tantalum powder were selected as the raw materials, with their morphology shown in Figure 1. The detailed particle size and compositions of the starting powders are summarized in Table 1. Alloys with the nominal compositions of Zr-25Ta-5Ti and Zr-30Ta respectively were made by mixing of the powders. To prevent contamination, the mixed powder was further blended in a planetary ball mill at 300 r/min for 5 h continuously with protection of Ar atmosphere. Stainless steel balls of 5 mm in diameter were selected and the mass ratio of the ball to the powder was 3:1. To facilitate the movement of the mixed powder during the sintering process and the easy removal of the sample after sintering, a coating layer of cubic boron nitride was applied on the inner surface of the graphite sheet as a thermal barrier layer, which inhibited the diffusion of carbon during sintering. The SPS sintering was performed at pressure/temperature of 40 MPa/1600 °C with a heating rate of 100 °C/min for a holding time of 15 min and the sizes of the sample was φ40 mm×7 mm.
2.2 Characterization
The density (ρ) and relative density (d=ρ/ρ0) of the produced Zr-Ta-Ti alloys were measured by Archimedes drainage method. The theoretical density (ρ0) of the Zr-Ta-Ti alloy is calculated as:

Figure 1 SEM micrographs showing morphologies of starting powders used in this work:

where w(A), w(B) and w(C) are the mass fractions of the elements A, B and C; ρA, ρB andρC are the theoretical densities of A, B, and C, respectively.
Table 1 Elemental compositions of Zr, Ta and Ti powders

The micro-hardness of Zr-Ta-Ti alloy samples was measured by Vickers micro-hardness tester. An indentation load of 1000 g was applied for a residence time of 15 s. Five measurements were taken for each sample and the average values of each sample were calculated.
The elastic modulus of the Zr-Ta-Ti alloys was measured by nanoindentation. An indentation load of 1000 g was applied for a residence time of 15 s. Five measurements were taken for each sample and the average and the standard deviation of each sample were calculated.
The phase compositions of the samples were determined using X-ray diffraction (XRD, D/Max-2550, Japan). The XRD testing was carried out at 40 kV with a scanning speed of 0.03(°)/s in a range of 2q from 25° to 85°. The microstructure and chemical compositions of the samples were analyzed by the Nano230 scanning election microscope (SEM) equipped with an energy dispersive X-ray spectrometer (EDS). Transmission electron microscopy (TEM) was conducted to determine the detailed microstructure of the Zr-Ta-Ti alloys. Thin foil specimens for the TEM analysis were prepared using focusing ion beam (FIB).
2.3 Electrochemical corrosion measurement
Sample of 6 mm×6 mm×6 mm was cut from the bulk materials by wire-electrode and then the sample was embedded in epoxy resin as working electrode. All the working electrodes were cleaned in alcohol with ultrasonic stirring after they were ground and polished. The electrochemical experiments were employed by the CHI 760 electrochemical workstation. Electrochemical tests were conducted in an artificial saliva whose composition was: 0.4 g/L NaCl, 0.4 g/L KCl, 0.795 g/L CaCl2·2H2O, 1 g/L urea, 0.78 g/L NaH2PO4·H2O, 0.005 g/L Na2S·2H2O. The pH value of this solution was 6.8, adjusting by adding NaOH. In addition, CP-Ti and Ti-6Al-4V alloy were used as control groups in this study.
In order to make the samples stable, the open-circuit potential (OCP) was measured firstly and the measurement time was 300 s. Then, the electrochemical impedance spectroscopy (EIS) was measured under open-circuit voltage, during which the frequency scanning range was from 10 kHz to 0.01 Hz and the amplitude was 10 mV. The measured data were analyzed by the ZSimDemo 3.30d software. Finally, the potentiodynamic polarization curve was measured in the range of -1.5 V to 1.0 V with the scanning speed of 1 mV/s.
The composition of the oxides on the surface of the Zr-Ta-Ti alloys was determined by X-ray photoemission (XPS) analysis where the 284.8 eV of C 1s peak was used to calibrate the binding energy. The morphology of electrochemically etched samples was observed by SEM.
3 Results
3.1 Raw materials and phase identification
Figure 1 shows the morphologies of the raw powders used in this study. The Zr, Ta and Ti powder particles are of irregular shapes. The mean particle sizes of the Zr, Ta and Ti powder are 11.86, 5.45 and 18.34 μm, respectively, as given in Table 1. Figure 2 shows the XRD patterns of the SPSed Zr-30Ta alloy and Zr-25Ta-5Ti alloy. It can be seen that the Zr-30Ta alloy is mainly composed of α+β phase, and Zr-25Ta-5Ti alloy consists of near β phase containing a small amount of α phase with the addition of Ti. It is well known that there are two kinds of phase structures of Zr including HCP structure (α-Zr) and BCC structure (β-Zr). Generally, Ti can lower the phase transition temperature of Zr, making α-Zr more easily convert to β-Zr, whilst Ta, as a β phase stabilizing element, can make β-Zr retain at room temperature [23].

Figure 2 X-ray diffraction patterns of Zr-Ta-Ti alloys
3.2 Microstructure characteristics
Figure 3 shows the microstructures of the Zr-Ta-Ti alloys. For Zr-30Ta alloy in Figure 3(a), the matrix phase is mainly composed of gray Zr-rich phase and the blocky white irregular phase is composed of Ta-rich phase in the grain boundaries. It is noted that there are un-solutionised point-liked Ta phase and Widmanstatten structure phase in the grains, as shown in the insert of Figure 3(a). Figure 3(b) shows the microstructure of Zr-25Ta-5Ti alloy. The microstructure displayed an obvious change with the addition of Ti. There are continuous Ta phases distributing along the grain boundaries and lamellar eutectoid with nanosized phases appearing inside the grain boundaries. In Figure 3(b), no blocky Ta-rich phase exists in Zr-25Ta-5Ti alloy, indicating that the diffusion is sufficient during the sintering process.

Figure 3 SEM images showing the microstructure of Zr-Ta-Ti alloys:
3.3 Mechanical properties
The relative density is 96% for Zr-30Ta alloy and increases up to 98% for Zr-25Ta-5Ti alloy with the addition of Ti, as shown in Figure 4(a). The relative density of Zr-25Ta-5Ti alloy is higher than that of Zr-30Ta alloy, which indicates that the addition of Ti is beneficial to the densification of the alloy. During the SPS sintering process, the Ti powder is in a molten state due to the high sintering temperature, and Ti promotes the diffusion of Ta powder during the flow, thereby promoting the densification. Figure 4(b) shows that the micro-hardness of Zr-Ta-Ti alloy is much higher than that of CP-Ti (about HV180). Generally, the higher the hardness of the alloy, the higher the wear resistance is. The hardnesses of Zr-30Ta alloy and Zr-25Ta-5Ti alloy are HV481 and HV454, respectively. Compared with Zr-30Ta alloy, the hardness of Zr-25Ta-5Ti alloy was decreased by 5.6%. The elastic modulus of Zr-Ta-Ti alloys obtained through nanoindentation test is shown in Figure 5. The elastic modulus of the Zr-30Ta alloy was (99.5±7.2) GPa, but it decreases to (73.6±6.3) GPa for Zr-25Ta-5Ti alloy.

Figure 4 Relative density and micro-hardness of Zr-Ta-Ti alloys
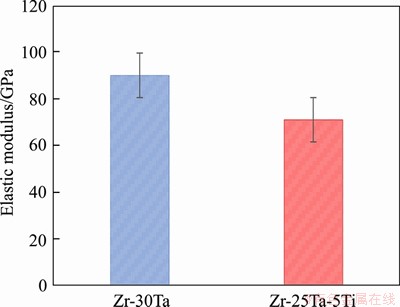
Figure 5 Elastic modulus of Zr-Ta-Ti alloys
3.4 Open-circuit potential (OCP)
Figure 6 shows OCP profiles of Zr-Ta-Ti, CP-Ti and Ti-6Al-4V alloys in artificial saliva. It is seen that the profiles for the Zr-Ta-Ti alloys were quite similar. In the initial stage, the potential was more negative, and then increased in the less negative direction and kept constant with further increasing time for Zr-25Ta-5Ti alloy. This implies that the passive films are spontaneously formed on the metal surface of the Zr-25Ta-5Ti alloy in artificial saliva. However, the profile of OCP changed slowly towards noble potentials for Zr-30Ta alloy during the whole immersion time. This could be ascribed to the high content of Ta in Zr-30Ta alloy, which makes the oxide film form on the surface of the alloy. It is noted that the final OCP values of CP-Ti, Ti-6Al-4V, Zr- 25Ta-5Ti and Zr-30Ta alloys were -0.602, -0.467, -0.431 and -0.428 V, respectively. It is indicated that the corrosion resistance of the Zr-Ta-Ti alloys has been improved in comparison to CP-Ti and Ti-6Al-4V alloys.

Figure 6 Open-circuit potential of Zr-Ta-Ti alloys, Ti-6Al-4V and CP-Ti
3.5 Potentiodynamic polarization
The potentiodynamic polarization curves of Zr-Ta-Ti, CP-Ti and Ti-6Al-4V alloys in artificial saliva are shown in Figure 7. The average Ecorr and Jcorr from the polarization curves calculated by chi760e software are shown in Table 2. The average values of Jcorr for all the samples were 5.88, 4.07, 1.26 and 0.46 μA/cm2 for CP-Ti, Ti-6Al-4V, Zr-25Ta-5Ti and Zr-30Ta alloys, respectively. The corrosion resistance can be sorted from high to low: Zr-30Ta alloy, Zr-25Ta-5Ti alloy, Ti-6Al-4V alloy, and CP-Ti.
3.6 Electrochemical impedance spectroscopy (EIS)
Figure 8 shows the EIS results of the alloys.

Figure 7 Anodic polarization curves recorded for Zr-Ta-Ti alloys, CP-Ti and Ti-6Al-4V
As indicated in the Nyquist plots (Figure 8(a)), the semicircular diameter of the Zr-Ta-Ti alloys was bigger than that of CP-Ti or Ti-6Al-4V alloy during the same immersion time. In the Bode plots (Figure 8(b)), the curves are composed of a linear variation and a flat part. The slope is close to -1 of the linear variation, which indicates the response of passive film capacitive behavior. The flat part of the high frequency (1-10 kHz) is related to the resistance of the electrolyte. The Bode-phase angle plots display a near-capacitive response with a phase angle of about -80° in the middle-frequency ranges, which indicates the present of the passive film on the samples surface [24, 25].
Table 2 Corrosion parameters of tested alloys obtained in artificial saliva


Figure 8 Nyquist spectra (a) and Bode spectra (b) for Zr-Ta-Ti alloys, Ti-6Al-4V alloy and CP-Ti in artificial saliva
In this study, an equivalent circuit model Rs(R1Q1) (R2Q2) with two time constants, is schematically shown in Figure 9. The EIS data of two passive films formed on the metal surface were fitted by ZSimpWin software [24-26]. The parameters Rs, R1 and R2 represent the electrolyte resistance, the resistances of the porous passive film and the dense passive film, respectively. Q is a constant phase element (CPE), which is used to replace capacitor to account for the imperfect capacitive behavior of the passivation film. The fitted parameters (Rs, Q1, n1, R1, Q2, n2 and R2) are listed in Table 3. It is obvious that the EIS data are in good agreement with the fitted data with the χ2 of about 10-3. The results indicated that the Zr-Ta-Ti alloys have a better corrosion resistance than CP-Ti and Ti-6Al-4V alloys, and Zr-30Ta alloy has an improved corrosion resistance in comparison to Zr-25Ta-5Ti alloy with 5 min immersion in artificial saliva.

Figure 9 Equivalent circuits for EIS
3.7 Surface analyses
The surface morphology of the corroded Zr-Ta-Ti alloys is shown in Figure 10. The alloys prepared by SPS contained a small amount of pores, and it is difficult to distinguish whether the pores are formed by pitting corrosion. It can be seen from the polarization curve that the samples did not undergo a significant activation before the test was stopped, indicating that pitting corrosion is probably not happening, or that the pitting is still in the nucleation stage. Figure 11 shows EDS analysis of the Zr-30Ta alloy after corrosion. The dark region (zone 3) has the highest oxygen content. This is because the content of Ta is less here and the corrosion potential is lower, which is easily corroded. It could also be resulted from the fact that Cl- destroys the passivation film on the surface of the alloy, causing the corrosive liquid to penetrate into the interior and contact the surface of the active metal substrate [26].
Table 3 Parameters of equivalent circuit for CP-Ti, Ti-6Al-4V and Zr-Ta-Ti alloys in artificial saliva


Figure 10 SEM images showing microstructure of Zr-Ta-Ti alloys after electrochemical corrosion:

Figure 11 Surface EDS analysis of Zr-30Ta alloy after corrosion
The XPS survey spectra at the surface of the Zr-Ta-Ti alloys after corrosion are shown in Figure 12. The XPS spectra from the Zr-Ta-Ti alloys after corrosion in artificial saliva are shown in Figure 13. The results demonstrate that the Zr 3d spectra consisted of 3d5/2 and 3d3/2 electron peaks, which belong to Zr4+ oxide state [26]. Ta 4f spectra exhibited a similar profile with 4f7/2 and 4f5/2 corresponding to Ta5+ oxide state [27]. The Ti 2p spectra consisted of 2p3/2 and 2p1/2 electron peaks, which were assigned to Ti4+ oxide state. The peaks at binding energy in O 1s spectra were assigned to O2- oxide state and absorbed H2O for Zr-25Ta-5Ti alloy [27-29].

Figure 12 XPS spectra for passive film formed on surface of Zr-Ta-Ti alloys
4 Discussion
4.1 Effect of titanium addition on microstructure evolution of Zr-Ta-Ti alloys
To understand the evolution of microstructure, the binary equilibrium phase diagrams of Zr-Ta and Zr-Ti are shown in Figure 14. For Zr-30Ta alloy, the eutectoid point is 1000 K, as indicated in Figure 14(a). The α phase can be transformed to β phase at 1823 K, and then β phase transfers into βZr phase and βTa phases, and βZr phase transfers into αZr phase during the cooling process. However, due to the short holding time during the SPS sintering, only part of α phase is converted into β phase, resulting in a large amount of α phase in Zr-30Ta alloy, which is consistent with the XRD results.

Figure 13 XPS spectra recorded at surface of Zr-Ta-Ti alloys:

Figure 14 Phase diagram of Zr-Ta (a) and Zr-Ti alloys (b)
Because of its high cooling rate, the β phase precipitates in the shape of needles inside the crystal grains. The diffusion coefficient of Ta in Zr is small at the low sintering temperature so that a large number of tantalum-rich phases appear in the microstructure.
In the phase diagram in Figure 14(b), Zr-Ti system shows a complete solid solution and the addition of Ti element can lower the phase transition temperature of Zr. It can be inferred that the phase transition temperature of Zr-30Ta alloy is about 1100 K, but the phase transition temperature of Zr-25Ta-5Ti alloy is about 1050 K. Therefore, β phase appears preferentially in the Zr-25Ta-5Ti alloy during the sintering process, and the decrease of the phase transition temperature also makes the β phase be retained at room temperature. The addition of Ti in Zr-25Ta-5Ti alloy promoted the diffusion of Zr and Ta in comparison to Zr-30Ta alloy. On the one hand, the addition of Ti promoted the diffusion of Zr atoms and increased the phase transition rate from α to β phase; a large amount of βZr phase was retained at room temperature in comparison to Zr-30Ta alloy. During the cooling process, part of the βZr phase transforms to βZr and βTa phase (βZr→βZr+βTa) and many needle-like precipitates appeared in the Zr-25Ta-5Ti alloy. Also, the addition of Ti promotes the diffusion of Ta atoms, therefore there is no apparently blocky rich-Ta phase. Furthermore, as the TEM images of Zr-25Ta-5Ti shown in Figure 15, there are three sets of diffraction patterns, which are βZr, βTa and ω phases, similar to the results reported by ZHAO et al [2]. The absence of ω phase in XRD results could be ascribed to the low content of ω phase in Zr-Ta-Ti alloy. It is reported that there are two ways of ω phase formation. One is the transformation of α phase to ω phase under high pressure; the other is the transformation of β phase to ω phase at high cooling rate [30-33]. In this study, the existence of ω phase may be related to the βZr→ωZr transition in the Zr-25Ta-5Ti alloy.
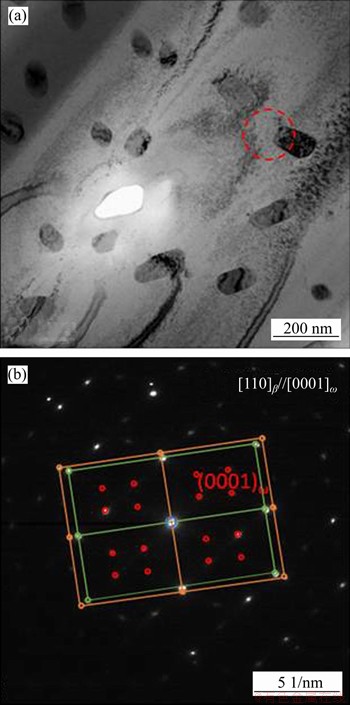
Figure 15 TEM image (a) and selected area electron diffraction pattern (SADPs) (b) of Zr-25Ta-5Ti alloy
4.2 Effect of titanium addition on mechanical properties of Zr-Ta-Ti alloys
The addition of Ti to Zr-25Ta-5Ti alloy produces solution strengthening. However, in most cases, the hardness of β phase is lower than that of α phase in Zr alloys, resulting in the lower hardness for Zr-25Ta-5Ti alloy. As well known, the low elastic modulus is an essential requirement for biomedical implant materials. Elastic modulus is the nature of matter itself, which is only related to the bonding force between atoms that is affected by crystal structure, composition and microstructure [34]. It has been documented in the literature that the addition of Ti to Zr alloy is beneficial to the reduction of the elastic modulus [35, 36]. Table 4 summarizes the mechanical properties of several typical Ti and Ti alloys [3, 37-39]. Zr-30Ta and Zr-25Ta-5Ti alloys have lower elastic modulus than the existing commercial CP-Ti and Ti-6Al-4V alloys. Moreover, compared to Zr-30Ta alloy, Zr-25Ta-5Ti alloy has a lower elastic modulus, which can be explained that the elastic modulus of the β-Zr is lower than that of the α-Zr [39-41]. The presence of β-Zr is beneficial to reduce the elastic modulus of the alloy. Zr-25Ta-5Ti alloy has more β-Zr phase than Zr-30Ta alloy, resulting in the reduced elastic modulus of Zr-25Ta-5Ti alloy.
Table 4 Mechanical properties of some implant materials

4.3 Effect of titanium addition on electrochemical behavior of Zr-Ta-Ti alloys
Compared with CP-Ti and Ti-6Al-4V alloys, Zr-Ta-Ti alloy exhibits an improved corrosion resistance. According to the potentiodynamic polarization curve in Figure 8, Zr-Ta-Ti alloys were passivated during the polarization process. The main components of the passivation film are ZrO2, Ta2O5 and TiO2. From the thermodynamic point of view, the Gibbs free energy of ZrO2, TiO2 and Ta2O5 are: -1042, -888.8 and -1910 kJ/mol, respectively. Ta2O5 passivation film is more effective to resistant corrosion than ZrO2 and TiO2 [42].
The corrosion resistance of the Zr-25Ta-5Ti alloy has a slightly reduction in comparison to Zr-30Ta alloy. The stability of the passivation film decreases with decreasing Ta content. On the other hand, the microstructure of Zr-25Ta-5Ti alloy contains a large number of fine needle-like phases, resulting in a relatively large density of grain boundaries, which could reduce its corrosion resistance [43]. It is reasonably presumed that the Ta-rich phase in Zr-25Ta-5Ti alloy is in contact with the Zr-rich phase to form a microscopic primary battery, and the Zr-rich phase is preferentially corroded.
Additionally, the anodic oxide film of Zr-Ta-Ti alloy has two layers of structure, consisting of a dense inner layer and a porous outer layer according to previous investigation [39]. The porous outer layer mainly is composed of ZrO2 and TiO2 and the inner layer mainly is composed of ZrO2, TiO2 and Ta2O5 due to the quick migration of Ti4+ and Zr4+ ions in the inner layer relative to that of Ta5+ ions [42].
5 Conclusions
1) Zr-30Ta alloy is a typical α+β dual phase alloy, and mainly consists of α-Zr, β-Ta and a small amount of β-Zr. The Zr-25Ta-5Ti alloy prepared by SPS is the near β type alloy, and the β phase mainly consists of β-Zr and β-Ta. There are a large number of acicular or lamellar Ta-rich phases inside the crystal grains in the Zr-Ta-Ti alloys, which are eutectoid structures precipitated under a rapid cooling rate.
2) The relative density of Zr-30Ta alloy reaches 96%, the hardness reaches HV 481 and the elastic modulus is about 99 GPa. With the addition of Ti, the relative density was increased to 98%, and the hardness and elastic modulus was decreased to HV 454 and 73 GPa for Zr-25Ta-5Ti alloy, respectively. Ti addition accelerated the desertification during the sintering process, and the increased β phase resulted in the decreased hardness and elastic modulus.
3) More stable ZrO2 and Ta2O5 generated in the passivation film on the surface of the Zr-Ta-Ti alloys are responsible for the improved corrosion resistance in comparison to CP-Ti and Ti-6Al-4V alloys during electrochemical corrosion. The addition of Ti slightly reduces the corrosion resistance of the Zr-Ta-Ti alloy due to the decrease in the more stable Ta2O5 passivation film.
References
[1] KONDO R. Microstructure and mechanical properties of as-cast Zr-Nb alloys [J]. Acta Biomaterialia, 2011, 7(12): 4278-4284. DOI: 10.1016/j.actbio.2011.07.020.
[2] ZHAO X L, LI L, NIINOMI M, NAKAI M, ZHANG D L, SURYANARAYANA C. Metastable Zr-Nb alloys for spinal fixation rods with tunable Young′s modulus and low magnetic resonance susceptibility [J]. Acta Biomaterialia, 2017, 62(15): 372-384. DOI: 10.1016/j.actbio.2017.08.026.
[3] ZHOU Y L, NIINOMI M, AKAHORI T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications [J]. Materials Science and Engineering A, 2004, 371(1, 2): 283-290. DOI: 10.1016/j.msea.2003.12.011.
[4] CATALANI S, STEA S, BERAUDI A, GILBERTI M E, BORDINI B, TONI A, APOSTOLI P. Vanadium release in whole blood, serum and urine of patients implanted with a titanium alloy hip prosthesis [J]. Clinical Toxicology, 2013, 51(7): 550-556. DOI:10.3109/15563650.2013.818682.
[5] MARECI D, BOLAT G, CAILEAN A, SANTANA J J, IZQUIERDO J, SOUTO R M. Effect of acidic fluoride solution on the corrosion resistance of ZrTi alloys for dental implant application [J]. Corrosion Science, 2014, 87(5): 334-343. DOI: 10.1016/j.corsci.2014.06.042.
[6] GODLEY R, STAROSVETSKY D, GOTMAN I. Corrosion behavior of a low modulus β-Ti-45%Nb alloy for use in medical implants [J]. Journal of Materials Science-Materials in Medicine, 2006, 17(1): 63-67. DOI: 10.1007/s10856- 006-6 330-6.
[7] MOLLER B, TERHEYDEN H, ACIL Y, PURCZ N M, HERTRAMPF K, TABAKOV A, BEHRENS E, WILTFANG J. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: An in vivo and in vitro study [J]. International Journal of Oral & Maxillofacial Surgery, 2012, 41(5): 638-645. DOI: 10.1016/j.ijom.2012.02.004.
[8] ZHOU F, WANG B, QIU K, LIN W, LI L, WANG Y B, NIE F. Microstructure, corrosion behavior and cytotoxicity of Zr–Nb alloys for biomedical application [J]. Materials Science and Engineering C, 2012, 32(4): 851-857. DOI: 10.1016/j.msec.2012.02.002.
[9] CORREA D R N, VICENTE F B, DONATO T A G, ARANA-CHAVEZ V E, BUZALAF M A R, Grandini C R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti-Zr system alloys for dental applications [J]. Materials Science and Engineering C, 2014, 34(1): 354-359. DOI: 10.1016/j.msec.2013.09.032.
[10] JHA S K, KESKAR N, VISHNU NARAYAN K I, MANI KRISHNA K V, SRIVASTAVA D, DEY G K, SAIBABA N. Microstructural and textural evolution during hot deformation of dilute Zr-Sn alloy [J]. Journal of Nuclear Materials, 2016, 482: 12-18. DOI: 10.1016/j.jnucmat.2016. 09.028.
[11] SUYALAT U, RYOTA K, YUSUKE T, HISASHI D, NAOYUKI N, TAKAO H. Effects of phase constitution on magnetic susceptibility and mechanical properties of Zr-rich Zr-Mo alloys [J]. Acta Biomaterialia, 2011, 7(12): 4259-4266. DOI: 10.1016/j.actbio.2011.07.005.
[12] CAI S, DAYMOND M R, KHAN A K, HOLT R A, OLIVER E C. Elastic and plastic properties of β-Zr at room temperature [J]. Journal of Nuclear Materials, 2009, 393(1): 67-76. DOI: 10.1016/j.jnucmat.2009.05.007.
[13] LIU J, CHANG L, LIU H, LI Y, YANG H, RUAN J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material [J]. Materials Science and Engineering C, 2017, 71: 512-519. DOI: 10.1016/j.msec.2016.10.043.
[14] BRANZOI I V, IORDOC M, CODESCU M. Electrochemical studies on the stability and corrosion resistance of new zirconium-based alloys for biomedical applications [J]. Surface and Interface Analysis, 2008, 40(3, 4): 167-173. DOI: 10.1002/sia.2750.
[15] BALLA V K, BODHAK S, BOSE S, BANDYOPADHYAY A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties [J]. Acta Biomaterialia, 2010, 6(8): 3349-3359. DOI: 10.1016/j.actbio. 2010.01.046.
[16] MEREIB D, SEU U C C, ZAKHOUR M, NAKHL M, JEAN-FRANCOIS S. Fabrication of biomimetic titanium laminated material using flakes powder metallurgy [J]. Journal of Materials Science, 2018, 53(5866): 1-12. DOI: 10.1007/s10853-018-2086-x.
[17] CHANG L, LIU J, YANG H, RUAN J. Effects of Zr contents on the microstructure, mechanical properties and biocompatibility of Ta-Zr alloys [J]. Materials Science Forum, 2018, 914: 37-44. DOI: 10.1016/j.matdes.2018. 107555.
[18] BAYODE B L, LETHABANE M L, OLUBAMBI P A, SIGALAS I, SHONGWE M B, RAMAKOKOVHU M M. Densification and micro-structural characteristics of spark plasma sintered Ti-Zr-Ta powders [J]. Powder Technology, 2017, 321: 471-478. DOI: 10.1016/j.powtec.2017.08.031.
[19] HENRIQUES V A R, SILVA C R M. Production of titanium alloys for medical implants by powder metallurgy [J]. Key Engineering Materials, 2001, 189-191: 443-448. DOI: 10.4028/www.scientific.net/KEM.189-191.443.
[20] CHAIM R. Densification mechanisms in spark plasma sintering of nanocrystalline ceramics [J]. Materials Science and Engineering A, 2007, 443: 25-32. DOI: 10.1016/ j.msea.2006.07.092.
[21] LIU H W, BISHOP D P, PLUCKNETT K P. Densification behaviour and microstructural evolution of Ti-48Al consolidated by spark plasma sintering [J]. Journal of Materials Science, 2017, 52(1): 613-627. DOI: 10.1007/ s10853-01 6-0358-x.
[22] OLIVEIRA N T C, BIAGGIO S R, NASCENTE P A P, ROCHA-FILHO R C, BOCCHI N. Investigation of passive films grown on biocompatible Ti-50Zr and Ti-13Zr-13Nb alloys by XPS [J]. Surface and Interface Analysis, 2006, 38(4): 410-412. DOI: 10.1002/sia.2201.
[23] MILSOEV, ZERJAV G, CALDERON MORENO J M, MONICA P. Electrochemical properties, chemical composition and thickness of passive film formed on novel Ti-20Nb-10Zr-5Ta alloy [J]. Electrochimica Acta, 2013, 99(3): 176-189. DOI: 10.1016/j.electacta.2013.03.086.
[24] PAN J, THIERRY D, LEYGRAF C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application [J]. Electrochimica Acta, 1996, 41(7): 1143-1153. DOI: 10.1016/0013-4686(95) 00465-3.
[25] DAI N, ZHANG L, ZHANG J, ZHANG X, NIE Q, CHEN Y, WU M, CHAO Y. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes [J]. Corrosion Science, 2016, 111: 703-710. DOI: 10.1016/ j.corsci.2016.06.009.
[26] JONASOVAL, MULLER F A, HELEBRANT A, STRNAD J, GREIL P. Biomimetic apatite formation on chemically treated titanium [J]. Biomaterials, 2004, 25(7): 1187-1194. DOI: 10.1016/j.biomaterials.2003.08.009.
[27] GUO W, SUN J, WU J. Electrochemical and XPS studies of corrosion behavior of Ti-23Nb-0.7Ta-2Zr-O alloy in Ringer’s solution [J]. Materials Chemistry and Physics, 2009, 113(2, 3): 816-820. DOI: 10.1016/j.m atchemphys.2008.08.043.
[28] LIN J, OZAN S, MUNIR K, WANG K, TONG X, LIY, LI G, WEN C. Effects of solution treatment and aging on the microstructure, mechanical properties, and corrosion resistance of a β type Ti–Ta–Hf–Zr alloy [J]. RSC Advances, 2017, 7(20): 12309-12317. DOI: 10.1039/c6ra28464g.
[29] MORENO J M C, VASILESCU E, DROB P, OSICEANU P, VASILESCU C, DROB S I, POPA M. Surface and electrochemical characterization of a new ternary titanium based alloy behaviour in electrolytes of varying pH [J]. Corrosion Science, 2013, 77(12): 52-63. DOI: 10.1016/ j.corsci.2013.07.026.
[30] HSIUNG L M, LASSILA D H. Shock-induced deformation twinning and omega transformation in tantalum and tantalum–tungsten alloys [J]. Acta Materialia, 2000, 48(20): 4851-4865. DOI: 10.1016/s1359-6454(00)00287-1.
[31] DEY G K, TEWARQI R, BANERJEE S, JYOTI G, GUPTA S C, JOSHI K D, SIKKA S K. Formation of a shock deformation induced ω phase in Zr20Nb alloy [J]. Acta Materialia, 2004, 52(18): 5243-5254. DOI: 10.1016/j.actamat. 2004.0 7.008.
[32] YEDDU H K, LOOKMAN T, SAXENA A. The simultaneous occurrence of martensitic transformation and reversion of martensite [J]. Materials Science and Engineering A, 2014, 594(594): 48-51. DOI: 10.1016/j.msea. 2013.11.036.
[33] ZHANAL P, HARCUBA P, HAJEK M, SMOLA B, STRASKY J, SMILAUEROVA J, VESELY J, JANECEK M. Evolution of ω phase during heating of metastable β titanium alloy Ti-15Mo [J]. Journal of Materials Science, 2018, 53(1): 837-845. DOI: 10.1007/s 10853-017-1519-2.
[34] HAO Y L, YANG R, NIINOMI M, KURODA D, ZHOU Y L, FUKUNAGA K, SUZUKI A. Aging response of the Young’s modulus and mechanical properties of Ti-29Nb-13Ta-4.6Zr for biomedical applications [J]. Metallurgical and Materials Transaction-Springer A, 2003, 34(4): 1007-1012. DOI: 10.1007/s1166 1-003-0230-x.
[35] WANG B, RUAN W, LIU J, ZHANG T, YANG H, RUAN J. Microstructure, mechanical properties, and preliminary biocompatibility evaluation of binary Ti-Zr alloys for dental application [J]. Journal of Biomaterials Applications, 2018, 33(6): 766-775. DOI: 10.1177/08853282188 11052.
[36] MURRAY J L. The Ti-Zr (titanium-zirconium) system [J]. Bulletin of Alloy Phase Diagrams, 1981, 2: 197-201. DOI: 10.1007/BF02881478.39.
[37] ASSIS S L, COSTA I. Electrochemical evaluation of Ti-13Nb-13Zr, Ti-6Al-4V and Ti-6Al-7Nb alloys for biomedical application by long-term immersion tests [J]. Materials and Corrosion, 2015, 58(5): 329-333. DOI: 10.1002/maco.200 604027
[38] YU Z. Evalution of haemocompatibility of TLM titaniun alloy with surfance herpairnization [J]. Rare Metal Materials and Engineering, 2009, 38(3): 932.
[39] CORNE P, MARCH P D, CLEYMAND F, GERINGER J. Fretting-corrosion behavior on dental implant connection in human saliva [J]. Journal of the Mechanical Behavior of Biomedical Materials, 2019, 94: 86-92. DOI: 10.1016/j. jmbbm.2019.02.025.
[40] NIE L, ZHAN Y, HU T, CHEN X, WANG C. β-type Zr-Nb-Ti biomedical materials with high plasticity and low modulus for hard tissue replacements [J]. Journal of the Mechanical Behavior Materials, 2014, 29: 1-6. DOI: 10.1016/j.jmbbm.2013.08.019.
[41] KONDO R, NOMURA N, SUYALATU, TSUTSUMI Y, DOI H, HANAWA T. Microstructure and mechanical properties of as-cast Zr-Nb alloys [J]. Acta Biomaterials 2011, 7(12): 4278-4284. DOI: 10.1016/j.actbio.2011.07.020.
[42] ZHOU Y L, NIINOMI M, AKAHORIT, FUKUI H, TODA H. Corrosion resistance and biocompatibility of Ti-Ta alloys for biomedical applications [J]. Materials Science and Engineering A, 2005, 398(1): 28-36. DOI: 10.1016/ j.surfcoat. 2005.04.044
[43] BOLAT G, IZQUIERDO J, SANTANA J J, MARECI D, SOUTO R M. Electrochemical characterization of ZrTi alloys for biomedical applications [J]. Electrochimica Acta, 2013, 106: 432-439. DOI: 10.1016/j.electacta.2012.10.026.
(Edited by YANG Hua)
中文导读
Ti对放电等离子烧结Zr-Ta-Ti合金组织、力学性能及耐蚀性的影响
摘要:本文研究了采用放电等离子烧结(SPS)技术制备的Zr-30Ta和Zr-25Ta-5Ti合金的组织、力学性能和耐蚀性。实验结果表明,采用SPS工艺制备的Zr-Ta-Ti合金孔隙率低,相对密度为96%~98%。XRD和TEM分析表明,Zr-30Ta合金由α+β相组成,Zr-25Ta-5Ti合金属于近β型合金,含有少量α和ω相。随着Ti的加入,合金的弹性模量由(99.5±7.2) GPa (Zr-30Ta)下降到(73.6±6.3) GPa (Zr-25Ta-5Ti)。此外,与CP-Ti和Ti-6Al-4V合金相比,Zr-Ta-Ti合金由于在合金表面钝化膜中生成更稳定的ZrO2和Ta2O5而具有更强的耐蚀性。以上研究表明,Zr-Ta-Ti合金是一种很有前途的新型金属生物材料。
关键词:放电等离子烧结;力学性能;显微组织;耐蚀性;锆合金
Foundation item: Project(51404302) supported by the National Natural Science Foundation of China; Project(QJ2018003A) supported by the Youth Scientific Research Foundation of the Central South University of Forestry and Technology, China
Received date: 2020-01-15; Accepted date: 2020-06-10
Corresponding author: YANG Hai-lin, PhD, Associated Professor; Tel: +86-731-88876644; E-mail: y-hailin@csu.edu.cn; MIAO Jing-lei, PhD; Tel: +86-731-88876644; E-mail: miaojinglei@126.com; ORCID: https://orcid.org/0000-0003- 3924-200X

