
ARTICLE
J. Cent. South Univ. (2019) 26: 304-311
DOI: https://doi.org/10.1007/s11771-019-4002-1

Equilibrium concentration of lithium ion in sodium aluminate solution
HUANG Wen-qiang(黄文强), LIU Gui-hua(刘桂华), LIU Peng(刘鹏), QI Tian-gui(齐天贵),
LI Xiao-bin(李小斌), PENG Zhi-hong(彭志宏), ZHOU Qiu-sheng(周秋生)
School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: Excess lithium in alumina is significantly bad for aluminum reduction. In this study, the concentration variation of lithium ion in sodium aluminate solution with addition of synthetic lithium aluminate was investigated. Elevating temperature, increasing caustic soda concentration, reducing alumina concentration or raising molar ratio ak improved equilibrium concentration of lithium ion in sodium aluminate solution. Agitation speed had a minimal effect on lithium ion concentration. Over 0.65 g/L lithium ion equilibrium concentration was observed in digestion process, whereas 35 mg/L lithium ion concentration remained in solution after precipitation time of 9 h. Moreover, equilibrium concentration decreased sharply from digestion of boehmite or diaspore to seed precipitation, about 95% lithium was precipitated into red mud (bauxite residue) and aluminum hydroxide. This study provides a valuable perspective in removal or extraction of lithium from sodium aluminate solution in alumina refineries.
Key words: lithium ion; equilibrium concentration; sodium aluminate solution; digestion; precipitation
Cite this article as: HUANG Wen-qiang, LIU Gui-hua, LIU Peng, QI Tian-gui, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng. Equilibrium concentration of lithium ion in sodium aluminate solution [J]. Journal of Central South University, 2019, 26(2): 304–311. DOI: https://doi.org/10.1007/s11771-019-4002-1.
1 Introduction
Diasporic bauxite in China contains high lithium contents. Consequently, more than 60% of smelter-grade alumina in China is rich in lithium (Li2O>0.03 wt%) [1], which increases lithium content (≥3 wt%) in electrolytes during aluminum reduction. Excess lithium in electrolytes not only decreases the melting point of electrolytes and the solubility of alumina, but also reduces current efficiency and increases energy consumption [2, 3]. However, the demand for lithium-bearing compounds, such as lithium carbonate, lithium hydroxide, lithium chloride, lithium bromide, and butyllithium, has significantly increased because of their extensive applications, including lithium ion batteries, glass, ceramics, and chemicals/ pharmaceuticals [4, 5]. In contrast to traditional lithium sources, including minerals/clays, brines, and spent lithium ion battery [4–6], lithium in industrial sodium aluminate solution is scarcely paid attention so far. Moreover, we found that lithium in sodium aluminate solution promoted the formation of the fine aluminum hydroxide in seed precipitation and reduced the strength of sandy alumina. Meanwhile, lithium was detected in red mud. As solid waste, red mud was treated by researchers with revegetation in a safe and effective way [7, 8]. The fine alumina and fragile alumina are detrimental to aluminum reduction. Therefore, removal or extraction of lithium from sodium aluminate solution in seed precipitation closely depends on the understanding of solubility of lithium aluminate in sodium aluminate solution.
The solubility of lithium aluminate hydrate in sodium aluminate solution has been rarely reported, although the solubility of lithium-bearing compounds in water has been extensively investigated [9–12]. The solubility of lithium hydroxide (<20 g/100 g H2O) in water depended on the temperature, ionic strength, and concentration of caustic soda [13–15]. However, the solubility of lithium aluminate hydrate in sodium aluminate solution is more complex because of high ionic strength, multiple components, and uncertain solution structure, which causes difficulty in examining the precipitation and solubility of lithium ion in sodium aluminate solution. STRATEN et al [16] found that lithium ion reacted with Al(OH)4- to form LiAl2(OH)2·2H2O and lithium ion promoted the precipitation and growth of bayerite in seed precipitation at 298 K. LI et al [17] reported that lithium ion did not affect particle size distribution of gibbsite and precipitation rate in seed precipitation, and that approximately 80% lithium precipitated into gibbsite after 4 h. VILYUGINA et al [18] suggested that the equilibrium concentration of lithium ion in sodium aluminate solution was related to the solubility of lithium aluminate hydrate (Li2O·2Al2O3·11H2O or Li2O·Al2O3·H2O). They also presented a formula for the equilibrium concentration of lithium ion with the addition of Li2O at 383 K to 553 K, but they did not describe the experimental procedures in detail. Errors may occur in the measurement of the equilibrium concentration of lithium ion in sodium aluminate solution because lithium aluminate hydrate is found to be readily precipitated from sodium aluminate solution in our experiment.
In this study, an online sample collection was employed at high pressure owing to high pressure. Effects of temperature, duration, concentration of caustic soda and alumina on the concentration of lithium ion added with synthetic lithium aluminate rather than LiOH or Li2O were discussed. This study provides a basis for the development of economical approach to efficiently remove or extract lithium from sodium aluminate solution.
2 Experimental procedure
2.1 Materials
A synthetic sodium aluminate solution was prepared with aluminum hydroxide, sodium hydroxide, and deionized water. A synthetic lithium aluminate from Li2CO3 and Al2O3 was used to prepare solution of sodium aluminate containing lithium ion because LiOH was readily dissolved into aluminate solution, but a stable clear solution in concentrated lithium ion concentration was difficultly obtained by addition of LiOH. All of aforementioned reagents were of analytical reagent grade (Sinopharm Group Co. Ltd) and used without further purification. αk is defined as the molar ratio of Na2O to Al2O3 in solution.
2.2 Preparation of lithium aluminate
Stoichiometric lithium aluminate hydrate is difficult to obtain because some lithium hydroxide can be dissolved in water. Therefore, lithium aluminate was prepared by sintering process in accordance with the following procedures [19, 20]. Li2CO3 and Al2O3 were fully mixed in 300 mL of deionized water at a 1:1 molar ratio with addition of 20 mL of 30% H2O2. The slurry was then dried at (373±5) K for 3 h. The resulting mixture was initially sintered at 1073 K for 4 h and then at 1273 K for 1 h in a muffle furnace. Afterwards, the mixture was cooled in the furnace, and synthetic lithium aluminate was obtained. Lithium aluminate contained α-LiAlO2 and γ-LiAlO2, as revealed by X-ray diffraction (XRD) pattern (Figure 1). The medium diameter d (0.5) of lithium aluminate was 80.308 μm.
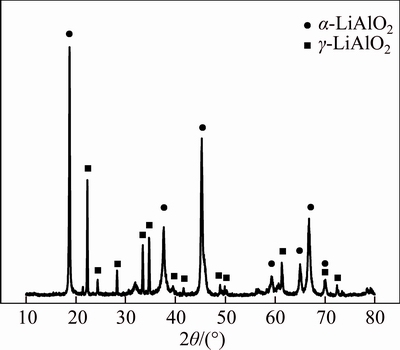
Figure 1 XRD pattern of lithium aluminate prepared in this experiment
2.3 Experimental procedure
Both lithium aluminate (10 g) and sodium aluminate solution (100 mL) were added to the reactor (Büchiglasuster bmd075 Switzerland) at high pressure. The reaction time was determined at 0 h when temperature was heated to the desired temperature. A filter with a diameter of 0.5 μm was used to collect the solution sample online at high pressure. Excess hydrochloric acid was then added to the filtrate and diluted to 100 mL. Similarly, lithium aluminate (10 g) and sodium aluminate solution (100 mL) were added to a stainless reactor (150 mL) bathed with glycerol at atmospheric pressure. The slurry was then quickly filtered in vacuum after the reaction was completed at atmospheric pressure, and the filtrate was diluted to 500 mL. The diluted solutions from high pressure or atmospheric pressure were used to determine the concentration of aluminum and lithium ion.
2.4 Methods
The concentration of alumina (Al2O3) and caustic soda (Na2O) in sodium aluminate solution was titrated [21]. The concentration of aluminum and lithium in the diluted filtrate was obtained by using an inductively coupled plasma emission spectrometer (ICP, Intrepid II XSP, USA). The alumina concentration was used as an internal standard to determine the concentration of lithium ion at high pressure because the concentration of alumina scarcely changed. XRD patterns were characterized by using an X-ray diffraction analyzer (D/MAAX2500, Japan). Particle size distribution was determined with Mastersizer 2000 (Malvern Instruments Ltd., UK).
3 Results and discussion
3.1 Concentration variation of lithium ion in sodium aluminate solution at high pressure
The temperature and concentration of Na2O for the analysis of lithium ion concentration variation were respectively determined at 433 to 513 K and 160 to 213 g/L on the basis of the digestion conditions of boehmite and diaspore by Bayer process.
3.1.1 Effect of reaction time
The equilibrium concentration of lithium in sodium aluminate solution is closely related to the dissolution rate of lithium aluminate because dissolution requires considerable time to reach equilibrium. Thus, the effect of duration on the concentration of lithium ion in sodium aluminate solutions was investigated (Figure 2).
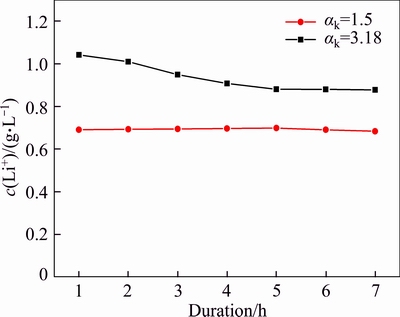
Figure 2 Effect of duration on concentration of lithium ion in sodium aluminate solution (T=453 K, agitation speed r=200 r/min, αk=3.18, c(Na2O)=213 g/L, and αk=1.5, c(Na2O)=205 g/L)
Figure 2 shows that the lithium ion concentration decreased slowly as the duration in the solution of αk=3.18 was prolonged until the concentration nearly reached a constant value after 4 h. By contrast, the lithium ion concentration almost approached a constant value in the solution of αk=1.50 from 1 to 7 h. These results suggest that lithium ion can easily reach its equilibrium concentration when lithium aluminate reacts with sodium aluminate solution. Therefore, the concentration of lithium ion at 6 h is determined as the equilibrium concentration in sodium aluminate solution in this work.
Figure 2 indirectly indicates that increase in alumina concentration or decrease in free caustic soda (caustic soda comprises NaOH and NaAl(OH)4, free caustic soda represents NaOH) reduced the lithium ion concentration.
3.1.2 Effect of agitation
Increasing agitation speed can promote mass transfer and then accelerate the reaction rate. The influence of agitation speed on lithium ion concentration in sodium aluminate solution at 453 to 513 K was investigated at agitation speeds of 200 and 400 r/min, respectively (Figure 3).
A similar variation curve for the concentration of lithium ion was observed in Figure 3 at an agitation speed of 200 or 400 r/min. This finding indicates that lithium ion concentration may be independent of agitation. Therefore, agitation speed is determined at 200 r/min in the subsequent experiments without regarding the effect of agitation on lithium ion concentration.
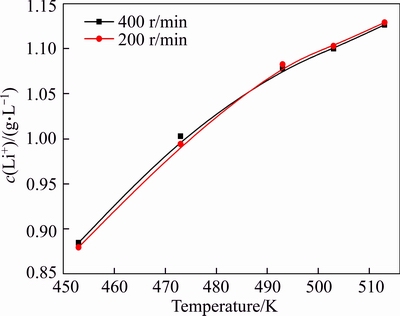
Figure 3 Effect of agitation on lithium ion concentration in sodium aluminate solution of αk=3.18, c(Na2O)=213 g/L for 6 h
Figure 3 also shows that increased temperature considerably raised the equilibrium concentration of lithium ions in sodium aluminate solution. The fact confirms that equilibrium concentration of lithium ion is sensitive to temperature. Thus, solutions should be collected online from reactors to accurately obtain the equilibrium concentration of lithium ion.
3.1.3 Effect of temperature
Temperature and molar ratio αk are different in each operation of the Bayer process. For example, temperature decreased from approximately 543 K in digestion to 323 K in seed precipitation. αk in solutions correspondingly reduced from about 3.0 to 1.4 in digestion and increased from 1.4 to 3.0 in seed precipitation. Figures 4(a) and (b) reveal the concentration variations of lithium ion at different temperatures.
In Figure 4(a), the concentration of lithium ion decreased slowly with duration in the solution of αk 3.18. The concentration of lithium ion also approached a constant value after 4 h. Meanwhile, elevating temperature also improved the concentration of lithium ion in sodium aluminate solution. All results indicate that high temperature corresponds to high lithium ion concentrations.
The concentration of lithium ion in the solution of αk=1.5 also reduced slightly with duration at different temperatures (Figure 4(b)). For example, the concentration of lithium ion decreased from 0.98 to 0.90 g/L at 0 and 4 h, respectively. After 4 h, duration slightly affected the concentration of lithium ion. Moreover, lithium ion concentration decreased notably when temperature varied at high temperature.
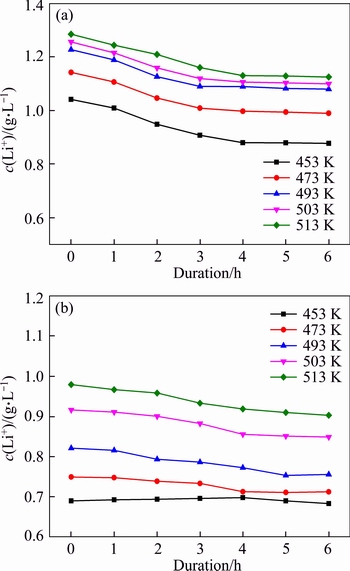
Figure 4 Effect of temperature and duration on concentration of lithium ion in different solutions of αk=3.18, c(Na2O)=213 g/L (a), and αk=1.50, c(Na2O)= 205 g/L at r=200 r/min (b)
Compared with data in Figures 4(a) and (b), the equilibrium concentration of lithium ion in a solution with a high molar ratio was greater than that in a solution with a low molar ratio. This finding suggests that the increase in alumina concentration is unfavorable for the increase in lithium ion concentration. With regard to variation of molar ratio in practical digestion, approximately 19.8% lithium will be lost into the red mud (bauxite residue) when the molar ratio varies from 3.18 to 1.5 during digestion of boehmite or diaspore at 513 K.
3.1.4 Effect of concentration of caustic soda
The concentration of caustic soda varies significantly during digestion, clarification, and seed precipitation. The effects of caustic soda concentration on lithium ion concentration are shown in Figure 5.
Figure 5 reveals that the increase in the concentration of caustic soda remarkably raised the lithium ion concentration. The temperature and concentration of caustic soda may synergistically affect lithium concentration because elevating temperature and concentration of caustic soda significantly promotes the lithium ion concentration in solution. For example, the concentration of lithium ion increased by 14.7% in the solution of c(Na2O)=160 g/L when the temperature increased from 453 to 513 K. By contrast, the concentration of lithium ion increases by 23.1% in the solution of c(Na2O)=213 g/L. A concentration gap was observed in the solution of c(Na2O)=213 g/L at 513 K and 160 g/L at 453 K. Therefore, considerable amount of lithium dissolved in solutions during Bayer digestion will remarkably precipitate into red mud in dilute operation for separation of sodium aluminate solution to the red mud. As 1000 kg Al2O3 can be produced from 2000–2500 kg bauxite, and 1000–1500 kg red mud is generated, lithium can be richened in red mud (bauxite residue) [22, 23].
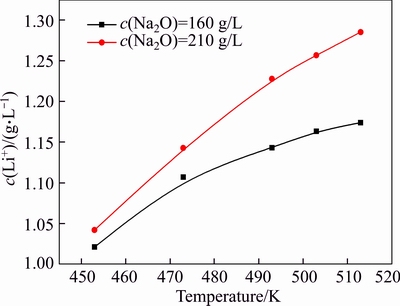
Figure 5 Effect of caustic soda concentration on concentration of lithium ion at different temperatures (αk=3.0, r=200 r/min, t=6 h)
Compared with the results described in a previous study [16], the equilibrium concentration of lithium ion in this study was greater by over 20% in the solution of αk=1.50. Moreover, a remarkable difference was found under the conditions of decrease in caustic soda concentration, increase in alumina concentration, and decrease in temperature. The errors were mainly attributed to significant variation of lithium ion concentration at different temperatures in solution, and concentration of lithium ion was sensitive to temperature. Therefore, online sampling is essential to accurately obtain the true equilibrium concentration of lithium aluminate hydrate under high pressure and elevated temperature.
3.1.5 Effect of alumina concentration
Figures 4 and 5 suggest that lithium ion concentration depends on the alumina concentration. Thus, the effects of alumina concentration in solution of c(Na2O)=213 g/L and c(Na2O)=160 g/L on lithium ion concentration are respectively presented in Figures 6(a) and (b).
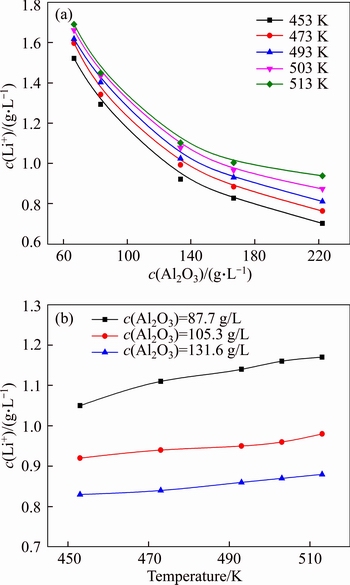
Figure 6 Effect of alumina concentration on lithium ion concentration in solution with c(Na2O)=213 g/L (a), and c(Na2O)=160 g/L (b) at 200 r/min and reaction for 6 h
The equilibrium concentration of lithium ion in the solution of c(Na2O)=213 g/L remarkably decreased when the alumina concentration increased, and equilibrium concentration of lithium ion reduced from 1.7 g/L to 0.7 g/L (Figure 6(a)). This fact may be due to the formation of lithium aluminate hydrate. The finding confirms that the lithium will be lost into red mud in digestion process. Figure 6(b) also reveals that the increase in the concentration of free caustic soda ([Na2O]- 0.5[NaAl(OH)4]) slightly increased the equilibrium concentration of lithium ion, possibly owing to the variation in activity coefficient.
3.2 Lithium ion concentration at atmospheric pressure in sodium aluminate solution
Clarification and seed precipitation are performed at 373 to 323 K (at atmospheric pressure). The co-precipitation of lithium aluminate hydrate directly contaminates aluminum hydroxide and may favor the formation of the fine aluminum hydroxide during seed precipitation. The precipitation of lithium aluminate hydrate depends on the equilibrium concentration of lithium ion. Therefore, the variation of lithium ion concentration at different durations is listed in Table 1 without addition of aluminum hydroxide seed at 338 to 368 K.
Table 1 Effect of duration on concentration of lithium ion at atmospheric pressure
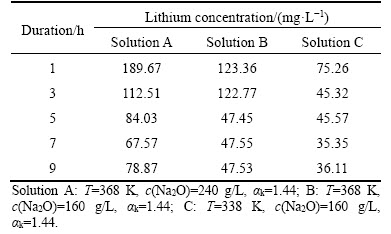
Less than 190 mg/L lithium ion concentration was found in Table 1, suggesting that lithium aluminate cannot be readily dissolved into aluminate solution. The fact also implies that equilibrium concentration of lithium ion in solution is remarkably low. Meanwhile, lithium ion concentration in Table 1 decreased sharply within 5 h. Afterwards, the lithium ion concentration almost remained unchanged after 5 h. Moreover, the decrease in caustic soda concentration and temperature favored the precipitation of lithium from sodium aluminate solution. In addition, the equilibrium concentration of lithium ion at 9 h was significantly higher than that reported by VILYUGINA et al [18]. The difference is maybe related to the difference in experimental materials, but the underlying explanation should be further discussed.
Table 1 also shows that high lithium ion concentration in solutions promoted lithium precipitation into aluminum hydroxide although temperature may be different. For example, the concentration of lithium ion (Solution B) decreased from 123.36 mg/L at 1 h to 47.53 mg/L at 9 h in 160 g/L Na2O solution with αk=1.44 at 368 K. As a result, 61.47% lithium was precipitated. By contrast, 52.02% of lithium was precipitated from 1 h to 9 h in 160 g/L Na2O solution with αk=1.44 at 338 K.
In addition, as the equilibrium concentrations of lithium ion during digestion at high pressure significantly differ from that during seed precipitation at atmosphere pressure, a great amount of lithium will be precipitated from solutions. Based on aforementioned data, the equilibrium concentration of lithium ion in sodium aluminate solution can reach over 1.2 g/L during digestion at high temperature in solution of αk=3.18. Afterwards, less than 130 mg/L lithium ion concentration was found in solution of c(Na2O)=160 g/L, αk=1.44. The facts imply that about 89% lithium likely precipitates into red mud as temperature decreases and the solution is diluted during clarification at about 368 K, after which, the lithium ion concentration further decreases to approximately 35 mg/L in seed precipitation at 338 K. Finally, approximately 5% lithium remains in the solution and 95% lithium will precipitate into red mud and aluminum hydroxide.
4 Conclusions
1) Equilibrium concentration of lithium ion varied under conditions of temperature of 453–513 K, αk of 1.5–3.18, Na2O concentration of 160–213 g/L. Over 0.65 g/L lithium ion equilibrium concentration was observed. Elevating temperature, increasing caustic soda concentration, reducing alumina concentration or raising molar ratio ak improved the lithium ion concentration in sodium aluminate solution. Agitation speed has a minimal effect on lithium ion concentration.
2) Lithium ion concentration also significantly decreased in seed precipitation. Concentrated lithium ion concentration led to a large amount of lithium precipitated into aluminum hydroxide from sodium aluminate solution. About 35 mg/L lithium ion concentration was observed after precipitation time of 9 h.
3) On the basis of variation of lithium ion concentration, about 89% lithium likely precipitates into red mud as temperature decreases and the solution is diluted during clarification at about 368 K. Moreover, about 95% lithium is precipitated into red mud and aluminum hydroxide from digestion to precipitation.
References
[1] HUANG Hai-bo, QIU Shi-lin. Influences of rich-lithium alumina on aluminum reduction production [J]. Light Metals, 2014(8): 26–28. (in Chinese)
[2] DANIELIK V, FELLNER P, THONSTAD J. Content of sodium and lithium in aluminium during electrolysis of cryolite-based melts [J]. Journal of Applied Electrochemistry, 1998, 28(11): 1265–1268.
[3] TABEREAUX A T, ALCORN T R, TREMBLEY L. Lithium-modified low ratio electrolyte chemistry for improved performance in modern reduction cells [M]// Essential Readings in Light Metals. Cham: Springer, 2016: 83–88.
[4] MESHRAM P, PANDEY B D, MANKHAND T R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review [J]. Hydrometallurgy, 2014, 150: 192–208.
[5] SWAIN B. Recovery and recycling of lithium: A review [J]. Separation and Purification Technology, 2017, 172: 388–403.
[6] CHAGNES A, POSPIECH B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries [J]. Journal of Chemical Technology and Biotechnology, 2013, 88(7): 1191–1199.
[7] XUE Sheng-guo, ZHU Feng, KONG Xiang-feng, WU Chuan, HUANG Ling, HUANG Nan, HARTLEY W. A review of the characterization and revegetation of bauxite residues (Red mud) [J]. Environmental Science and Pollution Research, 2016, 23(2): 1120–1132.
[8] ZHU Feng, CHENG Qing-yu, XUE Sheng-guo, LI Chu-xuan, HARTLEY W, WU Chuan, TIAN Tao. Influence of natural regeneration on fractal features of residue microaggregates in bauxite residue disposal areas [J]. Land Degradation and Development, 2018, 29(1): 138–149.
[9] FURUKAWA T, HIAKAWA Y, KONDO H, KANEMURA T. Dissolution behavior of lithium compounds in ethanol [J]. Nuclear Materials and Energy, 2016, 9: 286–291.
[10] FURUKAWA T, HIRAKAWA Y, KONDO H, KANEMURA T, WAKAI E. Chemical reaction of lithium with room temperature atmosphere of various humidities [J]. Fusion Engineering and Design, 2015, 98: 2138–2141.
[11] SILAMBARASAN A, RAJESH P, RAMASAMY P. Nucleation kinetics and growth aspects of negative solubility lithium sulphate monohydrate single crystal [J]. Journal of Crystal Growth, 2015, 409: 95–99.
[12] KIM T, OLEK J. The effects of lithium ions on chemical sequence of alkali-silica reaction [J]. Cement and Concrete Research, 2016, 79: 159–168.
[13] STEPHEN E F, MILLER P D. Solubility of lithium hydroxide in water and vapor pressure of solutions above 220° F [J]. Journal of Chemical and Engineering Data, 1962, 7(4): 501–505.
[14] MONNIN C, DUBOIS M. Thermodynamics of the LiOH+ H2O system [J]. Journal of Chemical and Engineering Data, 2005, 50(4): 1109–1113.
[15] PENSADO-RODRIGUEZ O, URQDIDI-MACDONALD M, MACDONALD D D. Electrochemical behavior of lithium in alkaline aqueous electrolytes. I. Thermodynamics [J]. Journal of the Electrochemical Society, 1999, 146(4): 1318–1325.
[16] van STRATEN H A, SCHOONEN M A A, de BRUVN P L. Precipitation from supersaturated aluminate solutions. III. Influence of alkali ions with special reference to Li+ [J]. Journal of Colloid and Interface Science, 1985, 103(2): 493–507.
[17] LI Xian-bin, LONG Zhi-yuan. Studies of the process for producing lithium-bearing alumina [J]. Mining and Metallurgical Engineering, 1989(3): 46–50. (in Chinese)
[18] VILYUGINA M D, MAKARENKOV V M, EREMIN N I. Lithium oxide solubility in aluminate solutions at elevated temperatures [J]. Izvestiya Vysshikh Uchebnykh Zavedenii, Tsvetnaya Metallurgia, 1983, 5: 72–74. (in Russian)
[19] CHENG Jian, GUO Lie-jin, XU Shi-sen, ZHANG Rui-yun. Submicron γ-LiAlO2 powder synthesized from boehmite [J]. Chinese Journal of Chemical Engineering, 2012, 20(4): 776–783.
[20] HEO S J, HU B, MANTHINA V, HILMI A, YUH C Y, SURENDRANATH A, SINGH P. Stability of lithium aluminate in reducing and oxidizing atmospheres at 700 °C [J]. International Journal of Hydrogen Energy, 2016, 41(41): 18884–18892.
[21] LIU Gui-hua, LI Zheng, QI Tian-gui, ZHOU Qiu-sheng, PENG Zhi-hong, LI Xiao-bin. Continuous changes in electrical conductivity of sodium aluminate solution in seeded precipitation [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4160–4166.
[22] XUE Sheng-guo, YE Yu-zhen, ZHU Feng, WANG Qiong-li, JIANG Jun, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276–286. DOI: 10.1016/j.jes.2018.10.010.
[23] ZHU Feng, LIAO Jia-xin, XUE Sheng-guo, HARTLEY W, ZOU Qi, WU Hao. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography [J]. Science of the Total Environment, 2016, 573: 155–163.
(Edited by YANG Hua)
中文导读
铝酸钠溶液中锂离子的平衡浓度
摘要:氧化铝中过量的锂不利于铝电解。本文研究了含锂铝酸钠溶液中锂离子平衡浓度的变化。研究表明,升高温度、增大苛性碱浓度、减小氧化铝浓度或升高苛性比值(ak)将有助于提高铝酸钠溶液中锂离子的浓度。搅拌速率对锂离子浓度影响很小。在拜耳溶出过程中,当铝酸钠溶液中锂离子平衡浓度超过0.65 g/L时,在晶种分解9 h后,铝酸钠溶液中锂离子浓度仅为35 mg/L。进一步研究发现,勃姆石或硬水铝石的溶出过程到晶种分解过程锂离子的平衡浓度急剧下降,大约有95%的锂会进入赤泥和氢氧化铝中。本文为氧化铝厂铝酸钠溶液中高效去除锂或回收锂提供了依据。
关键词:锂离子;平衡浓度;铝酸钠溶液;溶出;种分
Foundation item: Project(2015BAB04B01) supported by the National Key Technology R & D Program of China; Project(FA2017029) supported by Science and Technology Program of Chongzuo, China; Project(CSUZC201811) supported by the Open-End Fund for the Valuable and Precision Instruments of Central South University, China
Received date: 2018-11-10; Accepted date: 2018-12-12
Corresponding author: LIU Gui-hua, PhD, Professor; Tel/Fax: +86-731-88830453; E-mail: liugh303@csu.edu.cn; ORCID: 0000-0002- 9669-3774