
E-pH diagram of ZnS-H2O system during high pressure leaching of zinc sulfide
MU Wang-zhong(牟望重), ZHANG Ting-an(张廷安), LIU Yan(刘 燕), GU Yan(古 岩),
DOU Zhi-he(豆志河), L? Guo-zhi(吕国志), BAO Li(鲍 丽), ZHANG Wei-guang(张伟光)
Key Laboratory of Ecological Utilization of Multi-metal Intergrown Ores of Ministry of Education,
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 6 July 2009; accepted 16 January 2010
Abstract: The values of GΘ, EΘ or pH from 110 to 160 °C were calculated and the relevant potential expressions were obtained. E-pH diagrams of ZnS-H2O system at oxygen partial pressure of 0.8 MPa, ionic activity of 1.0 and different temperatures were drawn through thermodynamical calculation. With the temperature increasing, the stable regions of S and Zn(II) in the E-pH diagrams become gradually larger, but the amplification decreases over 150 °C. The impacts of leaching parameters, such as temperature, liquid to solid ratio, initial acidic concentration, leaching time, oxygen partial pressure and stirring speed on the leaching rate of Zn (II) and conversion rate of S in the single factor of high pressure leaching experiment of ZnS in autoclave, were studied. The leaching residue was examined by X-ray fluorescence (XRF) chemical composition identification and X-ray diffraction (XRD) phase identification, and the content of the leaching solution was tested by inductively coupled plasma-atomic emission spectrometry (ICP). The experimental results indicate that the leaching rate of zinc increases from 60.05% to 97.85% and the conversion rate of sulfur increases from 38.90% to 80.92% with the temperature increasing from 110 °C to 150 °C, 5:1 of liquid-to-solid ratio, 150 g/L of initial acidic concentration, 120 min of leaching time, 0.8 MPa of oxygen partial pressure, and 480 r/min of stirring speed, which tend to be stable over 150 °C. The experimental results correspond with theoretical calculation.
Key words: zinc sulfide; high pressure leaching; ZnS-H2O system; E-pH diagram
1 Introduction
Sulfides (sphalerite, marmatite) are predominant sources of zinc in the earth crust[1]. In the production of zinc from the ores, it can be used by pyrometallurgical method and hydrometallurgical method. Hydro- metallurgical method is more advantageous than pyrometallurgical method[2], but it has been reported that zinc metal produced from the concentrate was used by roast-leach-electrowinning process (RLE)[3]. The process involves a roasting step, which evolves toxic SO2 gas and requires a sulfuric acid plant to be set up in the smelter[4]. The prevention of SO2 emission from RLE and marketing of sulphuric acid are challenging the future of this technology[5]. As an environmental and economical technology, high pressure leaching is alternative to the traditional RLE route. In the latter, levels of SO2 emissions unacceptable for release to the environment have necessitated expensive gas cleaning equipment and production of sulfuric acid. However, in the high pressure leaching process, the sulfides are oxidized to elemental sulfur, which is recovered and stockpiled[6].
Leaching of zinc sulfide is based on the oxidation of zinc sulphide in an acidic environment[7]. In the high pressure leaching of ZnS, oxygen acts as an oxidant in the dissolution. The amount of oxygen in the solution has an important effect on the kinetics of the whole process[8]. High pressure leaching of ZnS is expressed by a simply basic reaction as follows:
ZnS+H2SO4+1/2O2=ZnSO4+S0+H2O (1)
E-pH diagram is a very important basis for thermodynamics of the high pressure leaching process. It also shows relations of the thermodynamic reactions and gives the reasonable conditions in the solution. But currently, it has few references about E-pH diagram of sulfide and water system at atmospheric pressure and room temperature (25 °C, 0.1 MPa), and the research at high temperature and pressure was rarely reported. WANG et al[9-10] only studied E-pH diagram of high iron sphalerite at 150 °C.
In this work, E-pH diagrams of ZnS system were drawn from 110 to 150 °C at oxygen partial pressure of 0.8 MPa and ionic activity of 1.0. Single factor experiments were performed to assess the influence of the parameters such as temperature, initial acidic concentration, oxygen partial pressure, liquid to solid ratio, leaching time and stirring speed.
2 Thermodynamic calculation
2.1 Method of drawing E-pH diagram
All hydrometallurgy reactions are expressed as
aA+mH++Ze=bB+cH2O (2)
According to Nernst isotherm equation, the equilibrium potential are expressed as
 (3)
(3)

 (4)
(4)
According to Nernst equation, ? =-RTlnK=
=-RTlnK=
 , whichever value of
, whichever value of  , K or
, K or  is known, E-pH diagram is obtained at this temperature according to Eq.(4). This reaction could be expressed as a linear relation in the E-pH diagram, whose slope is -2.303[RTm/(ZF)], intercept is
is known, E-pH diagram is obtained at this temperature according to Eq.(4). This reaction could be expressed as a linear relation in the E-pH diagram, whose slope is -2.303[RTm/(ZF)], intercept is  -2.303[RT/(ZF)]·lg[
-2.303[RT/(ZF)]·lg[ ], and the value of abscissa and ordinate of horizontal line are pH and j, respectively[11].
], and the value of abscissa and ordinate of horizontal line are pH and j, respectively[11].
2.2 Method of calculating  of materials
of materials
The methods such as the average of thermal melting method and the ionic similar to linear thermal melting method were used to calculate the thermodynamic data of each material during the high pressure leaching process of ZnS, but they are complicated and would easily be miscalculated[12].
YI[13] studied As-H2O system at high temperature. The following approximate calculation was obtained according to thermodynamics basic law as
 (5)
(5)
As the partial molar volume changes little below 300 °C,  could be neglected. When the change of temperature is not significant, cp could be approximately, so the Eq.(5) could be expressed as
could be neglected. When the change of temperature is not significant, cp could be approximately, so the Eq.(5) could be expressed as
 (6)
(6)
According to the Eq.(7),  could be expressed as Eq.(8). Then Eq.(8) is substituted into Eq.(6), so the Eq.(9) is obtained:
could be expressed as Eq.(8). Then Eq.(8) is substituted into Eq.(6), so the Eq.(9) is obtained:
 (7)
(7)
 (8)
(8)
 (9)
(9)
 of ions is calculated at high temperature, according to “corresponding principle of entropy” proposed by CRISS and COBBLE[14] and empirical formula for calculating the absolute entropy proposed in Ref.[15], so Eq.(10) is obtained:
of ions is calculated at high temperature, according to “corresponding principle of entropy” proposed by CRISS and COBBLE[14] and empirical formula for calculating the absolute entropy proposed in Ref.[15], so Eq.(10) is obtained:
 (10)
(10)
where aT =A(T-298), dT=D(T-298) and cT=1-0.002 24(T- 298).
In Eq.(10), Z is the absolute value of ion charge number. Compared with “corresponding principle of entropy”, this empirical formula adds dTZ to adjust the influence of ionic charge.
For the convenience of calculation, Eq.(10) is substituted into Eq.(9), and Eq.(11) is obtained:
The values of A, D, α, β and γ are listed in Table 1 and Table 2[13]. The values from Table 1 and Table 2 are substituted into Eq.(11), then  of main materials in ZnS-H2O system is listed in Table 3.
of main materials in ZnS-H2O system is listed in Table 3.
 (11)
(11)

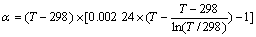 (12)
(12)
 (13)
(13)
 (14)
(14)
Table 1 A and D values for calculation of 

Table 2 α, β and γ values for calculation of 

Table 3  of main substances of ZnS-H2O system at different temperatures
of main substances of ZnS-H2O system at different temperatures

3 Drawing E-pH diagram of ZnS-H2O system at high temperature and high pressures
The data used in thermodynamic calculation are from Refs.[16-17], and the calculating thermodynamic data refer to Table 3. According to the method above,
 is calculated by
is calculated by
 (16)
(16)
Substituting  into Nernst formula, then the expressions of
into Nernst formula, then the expressions of  or
or  are acquired at different
are acquired at different
temperatures. The expressions are listed in Table 4. Submitting them into Eq.(4), then the E-pH diagrams of ZnS-H2O system (Fig.1) were drawn from 110 to 160 °C at oxygen partial pressure of 0.8 MPa and activity of 1.0.
Corresponding to the material of reactions (18) and (19), the pH value of stable region is beyond reaction (20), so the two reactions could not be expressed in Fig.1. The stable region of Zn(II) and S is determined from the E-pH diagram of ZnS-H2O system, and the leaching conditions are controlled to ensure that ZnS converts to zinc ionic and sulfide. In this work, E-pH diagrams of different temperatures are compared in the same activity and oxygen partial pressure, and how the stable region of zinc ion changes could be observed.
According to Fig.1 and Fig.2, with increasing the temperature, the stable region of S and Zn(II) does not change significantly while the deposition potential of corresponding reaction in stable region increases. But when the temperature is higher than 150 °C, the amplification becomes smaller. According to  the improvement of deposition potential means that the value of ?GT is more negative. That is to say, the higher the temperature is, the more thoroughly the reaction proceeds. This shows that increasing temperature is beneficial to the process of leaching and the conditions of reaction kinetics are more favorable at high temperature.
the improvement of deposition potential means that the value of ?GT is more negative. That is to say, the higher the temperature is, the more thoroughly the reaction proceeds. This shows that increasing temperature is beneficial to the process of leaching and the conditions of reaction kinetics are more favorable at high temperature.
Table 4 Calculated values of each reaction of  or
or  of ZnS-H2O system at different temperatures
of ZnS-H2O system at different temperatures


Fig.1 E-pH diagrams of ZnS-H2O system at different temperatures with activity of 1.0 and oxygen pressure of 0.8 MPa: (a) 110 °C; (b) 120 °C; (c) 130 °C; (d) 140 °C; (e) 150 °C; (f) 160 °C

Fig.2 Comparison of S-quality regional at different temperatures and activity of 1.0 and oxygen pressure of 0.8 MPa ((b) is enlargement of (a))
4 Experimental
4.1 Material
The experimental material was ZnS (analytically pure), and the additive is calcium lignosulfonate, and leaching solution is H2SO4 (98%) diluted to a certain concentration.
4.2 Leaching experiment
The major equipment used in the experiment was a 2 L zirconium autoclave (KCFD2-10 type) consisting of a reactor chamber and a head assembly, both of which could be combined and tightened with a leak-proof closure. Heat was provided to the autoclave by an electrical heating mantle that was connected to a thermocouple and controlled by an external temperature controller. The autoclave was further equipped with a solid feeding device that allowed for convenient injection of zinc sulfide or sulphuric acid into the reactor chamber once the desired temperature had been reached. The autoclave was directly connected to an oxygen cylinder in order to reach the required oxygen partial pressure[18].
The experimental parameters are 110-160 °C of the leaching temperature, 0.2-1.0 MPa of oxygen partial pressure, 1?1-8?1 of liquid to solid ratio, 120-160 g/L of initial acidic concentration, 30-150 min of leaching time and 120-600 r/min of stirring speed.
4.3 Analysis
The Prodigy XP type inductively coupled plasma-atomic emission spectrometry (ICP) of American Leeman Lab was used to test the Zn2+ content of leaching solution. The chemical composition identication of leach residue was analyzed by ZSX 100e X-ray fluorescence spectrometer (XRF) and phase identication was analyzed by PW3040/60 X-ray diffraction (XRD) of Dutch Panalytical Company, and the diffraction angle (2θ) was from 5° to 90°, and scanning rate was 7(°)/s.
5 Results and discussion
The E-pH diagrams of ZnS-H2O system are used to guide the single factor experiment of ZnS high pressure leaching. The XRD pattern of leaching residue is shown in Fig.3 and the experimental results are shown in Fig.4.
According to E-pH diagram, temperature is the most important factor in the process of leaching. In the temperature range from 110 to 150 °C, the leaching rate of zinc increases from 60.05% to 97.85% and the conversion rate of sulfur increases from 38.90% to 80.92% when other conditions are 5?1 of liquid-to-solid ratio, 150 g/L of initial acidic concentration, 120 min of leaching time, 0.8 MPa of oxygen partial pressure, and 480 r/min of stirring speed. The experimental results tend to be stable over 150 °C. With the temperature increasing from 110 to 150 °C, the leaching of Zn(II) and the conversion of S proceed more thoroughly. It is favorable for kinetics condition at high temperature, and temperature has an important effect on decreasing the kinetics resistance.

Fig.3 XRD pattern of leaching slag
In the process of ZnS high pressure leaching, oxygen acts as an oxidant in the dissolution, passes through gas/liquid interphase firstly and is adsorbed on the surface of solid particles. Then, the oxygen molecule which is adsorbed cracks into oxygen atom, and it is the key link of the whole oxidation process. However, this cracking reaction needs high activation energy. Consequently, temperature has a significant influence on promoting the oxygen bond fracture and making the oxygen molecule dissoluted crack into oxygen atom. Meanwhile, effect of temperature could be expressed by reaction rate constant. According to Arrhenius formula K=Aexp[-E/(RT)], the value of K increases and the motion of ion aggravates with increasing the temperature, which is beneficial to improving the reaction rate. In conclusion, the experimental results correspond with theoretical calculation.
6 Conclusions
1) E-pH diagrams of ZnS-H2O system were drawn by thermodynamic calculation method under the condition of oxygen partial pressure of 0.8 MPa, ionic activity of 1.0 and different temperatures from 110 to 160 °C.

Fig.4 Single factor experimental results of ZnS high pressure leaching
2) By comparing the change trend of the stable region of S and Zn(II) from 110 to 160 °C, it is shown that with increasing the temperature, ?GT of reactions corresponding to S and Zn(II) stable regions in the E-pH diagrams are more negative, and the values of pH increase. But, when the temperature is higher than 150 °C, the amplification is smaller. Thus, how temperature influences the stable region is clear, and E-pH diagram provides thermodynamics basis for ZnS high pressure leaching.
3) The experimental results show that with increasing the temperature from 110 to 150 °C, while other parameters are 5?1 of liquid to-solid-ratio, 150 g/L of initial acidic concentration, 120 min of leaching time, 0.8 MPa of oxygen partial pressure, and 480 r/min of stirring speed, the leaching rate of zinc increases from 60.05% to 97.85% and the conversion rate of sulfur increases from 38.90% to 80.92%, which tend to be stable over 150 °C. The experimental results of ZnS high pressure leaching justify the guiding significance of E-pH diagram.
References
[1] PECINA T, FRANCO T, CASTILLO P, ORRANTIA E. Leaching of a zinc concentrate in H2SO4 solutions containing H2O2 and complexing agents [J]. Minerals Engineering, 2008, 21: 23-30.
[2] ?OPUR M, ?ZMETIN C, ?ZMETIN E, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulfate acid solutions [J]. Chemical Engineering and Processing, 2004, 43: 1007-1014.
[3] SOUZA A D, PINA P S, LEAO V A, SILVA C A, SIQUERIRA P F. The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate [J]. Hydrometallurgy, 2007, 89: 72-81.
[4] SAHU S K, SAHU K K, PANDEY B D. Leaching of zinc sulfide concentrate from the ganesh-himal deposit of it [J]. Metallurgical and Materials Transactions B, 2006, 37: 541-549.
[5] DELLER G. World zinc supply and demand-heading for a late decade price spike [C]//Symposia of TMS Lead & Zinc. Kyoto, 2005: 17-25.
[6] BALDWIN S A, DEMOPOULOS G P, PAPANGELAKIS V G. Mathematical modeling of the zinc pressure leach process [J]. Metallurgical and Materials Transactions B, 1995, 26: 1035-1047.
[7] HAAKANA T, LAHTINEN M, TAKALA H, RUONALA M, TURUMEN I. Development and modelling of a novel reactor for direct leaching of zinc sulphide concentrates [J]. Chemical Engineering Science, 2007, 62: 5648-5654.
[8] KASKIALA T. Determination of mass transfer between gas and liquid in atmospheric leaching of sulphidic zinc concentrates [J]. Minerals Engineering, 2005, 18: 1200-1207.
[9] WANG Ji-kun, LI Cun-xiong, LI Yong, ZHANG Hong-yao, HUANG Hui, YAN Jiang-feng, LIU Lu, WEI Chang. The ε-pH diagram of ZnS-FeS-H2O system during acid leaching under pressure of high iron sphalerite [J]. Nonferrous Metals (Extractive Metallurgy), 2006(2): 2-5. (in Chinese)
[10] WANG Ji-kun, ZHOU Ting-xi. The technology and Industrialization of zinc concentrates oxygen pressure leaching [M]. Beijing: Metallurgical Industry Press, 2005: 45-128. (in Chinese)
[11] LI Zhi-qiang, HE Liang-hui. Diagrams of chemical potential and application in aqueous solutions [M]. Chengdu: Chengdu Science and Technology Press, 1991: 94-160. (in Chinese)
[12] JIN Zhen-nan, JIANG Kai-xi, WEI Xu-jun, WANG Hai-bei. Potential-pH diagrams of As-S-H2O system at high temperature [J]. Mining and Metals, 1999, 8 (4): 45-50. (in Chinese)
[13] YI Xian-wu. An empirical estimation of standard entropy  for some complex cations and the ε-pH diagrams of As-H2O system at elevated temperature [J]. Journal of Kunming University of Science and Technology, 1982, (3): 58-73. (in Chinese)
for some complex cations and the ε-pH diagrams of As-H2O system at elevated temperature [J]. Journal of Kunming University of Science and Technology, 1982, (3): 58-73. (in Chinese)
[14] CRISS C M, COBBLE J W. The thermodynamic properties of high temperature aqueous solutions IV: Entropies of the ions up to 200° and the correspondence principle [J]. Journal of the American Chemical Society 1964, 86(24): 5385–5390.
[15] ХОДАКОВСКИЙ И Л. Migration form of tungsten in hydrothermal [J]. Journal of Chemistry, 1968(12): 1486-1503.
[16] FU Chong-yue. thermodynamic data in aqueous solutions at high temperature [M]. Beijing: Metallurgical Industry Press, 1983: 523-674. (in Chinese)
[18] FILIPPOU D, KONDURU R, DEMOPOULOS G P. A kinetic study on the acid pressure leaching of pyrrhotite [J]. Hydrometallurgy, 1997, 47: 1-18.
(Edited by LI Xiang-qun)
Foundation item: Project (2007CB613504) supported by the National Basic Research Program of China; Project(20050145029) supported by the PhD Programs Foundation of Ministry of Education of China; Project(2005221012) supported by the Science and Technology Talents Fund for Excellent Youth of Liaoning Province, China
Corresponding author: ZHANG Ting-an; Tel: +86-24-83687732; E-mail:mwz_01@163.com
DOI: 10.1016/S1003-6326(09)60410-6