硫酸铵加压热解-酸浸工艺提取含钛高炉渣中有价金属
来源期刊:中国有色金属学报(英文版)2020年第10期
论文作者:边振忠 冯雅丽 李浩然
文章页码:2836 - 2847
关键词:含钛高炉渣;硫酸铵;加压热解;高值化产品
Key words:Ti-bearing blast furnace slag; ammonium sulfate; pressurized pyrolysis; high value products
摘 要:提出一种硫酸铵(AS)加压热解-酸浸新工艺,从含钛高炉渣中(TBBF)分段回收有价金属。结果表明,当在铵矿比3:1、370 °C的条件下热解90 min时,钛、铝和镁的提取率分别为94.5%、91.9%和97.4%。酸浸液在沸腾状态下重结晶除杂可获得TiO2含量为94.1%的钛产品。上述结晶母液分别调节pH=6和pH≥12.2,可获得合格的氧化铝和氧化镁产品。XRD和SEM-EDS分析表明,焙烧样品中主要物相为NH4AlSO4、CaSO4 和TiOSO4。热力学分析显示,在最优条件下,原矿中钙钛矿、镁铝尖晶石和透辉石能与AS产生的中间产物自发反应。
Abstract: A novel method of extracting valuable metals from Ti-bearing blast furnace slag (TBBF slag) via pressure pyrolysis of recyclable ammonium sulfate (AS)-acid leaching process was proposed. The results show that when pressurized roasting at an AS-to-slag mass ratio 3:1 and 370 °C for 90 min, the extraction rates of titanium, aluminum and magnesium reached 94.5%, 91.9% and 97.4%, respectively. The acid leaching solution was subjected to re-crystallization in a boiling state to obtain a titanium product having a TiO2 content of 94.1%. The above crystallization mother liquor was adjusted to pH=6 and pH≥12.2, respectively, and then qualified Al2O3 and MgO products were obtained. The analysis through XRD and SEM-EDS proves that the main phases in roasted samples were NH4AlSO4, CaSO4 and TiOSO4. The thermodynamic analysis presents that the main minerals of perovskite, spinel and diopside in raw ore could spontaneously react with the intermediate produced by AS under optimal conditions.
Trans. Nonferrous Met. Soc. China 30(2020) 2836-2847
Zhen-zhong BIAN1, Ya-li FENG1, Hao-ran LI2
1. School of Civil and Resource Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received 31 December 2019; accepted 18 June 2020
Abstract: A novel method of extracting valuable metals from Ti-bearing blast furnace slag (TBBF slag) via pressure pyrolysis of recyclable ammonium sulfate (AS)-acid leaching process was proposed. The results show that when pressurized roasting at an AS-to-slag mass ratio 3:1 and 370 °C for 90 min, the extraction rates of titanium, aluminum and magnesium reached 94.5%, 91.9% and 97.4%, respectively. The acid leaching solution was subjected to re-crystallization in a boiling state to obtain a titanium product having a TiO2 content of 94.1%. The above crystallization mother liquor was adjusted to pH=6 and pH≥12.2, respectively, and then qualified Al2O3 and MgO products were obtained. The analysis through XRD and SEM-EDS proves that the main phases in roasted samples were NH4AlSO4, CaSO4 and TiOSO4. The thermodynamic analysis presents that the main minerals of perovskite, spinel and diopside in raw ore could spontaneously react with the intermediate produced by AS under optimal conditions.
Key words: Ti-bearing blast furnace slag; ammonium sulfate; pressurized pyrolysis; high value products
1 Introduction
In China, about 90% of the titanium reserves in the western Panxi regions exist in the form of the vanadium-bearing titanomagnetite, which is known for the most titanium resources in the world [1,2]. Ti-bearing blast furnace (TBBF) slag is formed by natural cooling or water quenching of slag with high melting temperature after blast furnace smelting [3]. The content of TiO2 in TBBF slag is generally above 20% [4]. Meanwhile, TBBF slag contains abundant aluminum and magnesium, which is widely used in coated steel [5], nanocomposite [6] and catalysts [7]. Nevertheless, titanium, magnesium and aluminum in TBBF slag have complex inlay relationship and very fine particle size, which makes it difficult to separate and recover them by conventional flotation or magnetic separation techniques. At present, TBBF slag has already accumulated 60 million tons, and it is still increasing at a rate of 3 million tons per year. Most of TBBF slag is simply stacked under outdoor condition, resulting in waste of resources and environment pollution [8]. Hence, it is urgent for TBBF slag to develop an efficient and feasible process of recover titanium, aluminum and magnesium.
To recover valuable metals in TBBF slag, the technology of high temperature carbonization-low temperature chlorination is widely applied to extracting titanium in pyrometallurgy. It is reported that the carbonization rate and chlorination rate of titanium are both above 85% [9]. The titanium mineral in TBBF slag is firstly reduced to TiC under high temperature, and then recovering titanium by chlorination to form TiCl4 which can be reduced by magnesium metal to obtain sponge titanium [10,11]. Owing to the chlorination reaction rate of TiC is much higher than the rate of other oxide impurities, the technology mainly achieves the purpose of selectively allowing the TiC to be chlorinated and the other impurities to remain in the slag [12-14]. However, the carbonization temperature is usually controlled between 1600 and 1800 °C in a closed electric furnace which requires higher refractory materials [15,16]. Meanwhile, this process has deficiencies involving Cl2 and HCl gases which can corrode the equipment and the titanium tailings contain a large amount of chloride causing secondary pollution [17].
Up to now, process routes that use ammonium sulfate ((NH4)2SO4, AS) or ammonium bisulfate (NH4HSO4, ABS) as reagents for the extraction step are gaining more research attention [18]. The thermodynamic decomposition of AS was reported earlier [18-23], as shown in Fig. 1. It can be seen that ABS and aminosulfonic acid (NH2SO3H, ASA) will form upon heating of AS above 100 °C. Triammonium hydrogen disulfate ((NH4)3H(SO4)2, AHS) and ammonium pyrosulphate ((NH4)2S2O7, APS) will be synthesized as the third intermediate with these two decomposition reactions, which means that several substances have no strict boundaries between their production and transformation and can coexist.
AS roasting is a potential cleaner production process with low energy consumption and high selectivity [24]. ZHANG [25] reported the ammonium sulphate and potassium sulphate melting method to recover titanium from TBBF slag with 94.7% of recovery efficiency. WANG et al [26] also reported a novel route for indirect mineral carbonation of TBBF slag through the recyclable AS roasting, showing the sulfation ratios of calcium, magnesium, titanium and aluminium were 83%, 92.6%, 87% and 84.4%, respectively. In this roasting process, the metal oxides and their sulfides can be converted into water-soluble metal sulfates to achieve the extraction and separation of various valuable metals. Nevertheless, decomposition reaction of ASA occurs at above 205 °C, releasing N2, NH3, SO3, H2O and H2SO4, which is unfavorable to the roasting process and will lead to a low selective conversion of valuable metal titanium, aluminum and magnesium in minerals into soluble salts.
Here, considering the superior activation during ammonium sulfate roasting to treat TBBF slag and its potential application as an efficient additive, this study focused on: (1) evaluating the technical feasibility of simultaneous extraction of titanium, aluminum and magnesium by AS pressurized pyrolysis-acid leaching processes and probing the influence of closed environment on the extraction rate of titanium, aluminum and magnesium; (2) investigating stepwise recovery of titanium, aluminum and magnesium high value products and identifying the process of AS efficient recycling; (3) proposing the mechanism for thermal decomposition of TBBF slag with AS.

Fig. 1 Thermal decomposition mechanism of AS
2 Experimental
2.1 Materials
The TBBF slag used in this research was received from Panzhihua Iron and Steel Co., Ltd., (Sichuan, China). The chemical composition analyzed using multiphase X-ray fluorescence (XRF) is shown in Table 1. The contents of TiO2, Al2O3 and MgO reach 27.2%, 11.4% and 7.15%, respectively, indicating high recycling value. The mineral composition was revealed by X-ray powder diffraction (XRD) (Fig. 2), showing that dominant minerals were perovskite (CaTiO3), diopside (CaMg(SiO3)2) and magnesium aluminium spinel (MgAl2O4). All the reagents used in the work were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd.
Table 1 Main chemical composition of TBBF slag (wt.%)

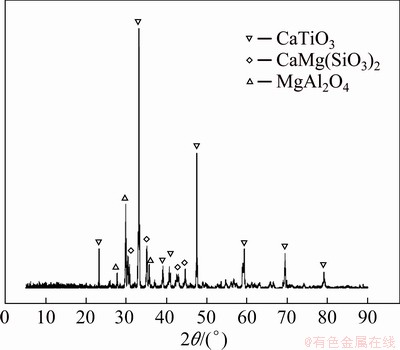
Fig. 2 XRD pattern of TBBF slag
2.2 Experimental procedure
2.2.1 AS roasting
The TBBF slags were firstly ground by the rod mill to the fineness of <0.074 mm passing 74.6% and dried overnight at 90 °C. 20 g TBBF slags were thoroughly mixed with AS in a designated dosage. The mixture was placed in a reaction kettle (TGYFA, Zhengzhou Yuda Instrument Co., Ltd., Zhengzhou, China) equipped with 0.3 L titanium alloy lining, heated at a rate of 6 °C/min to the required temperature (290-450 °C), and then roasted for 0.5-4 h at the stirring speed of 360 r/min. Meanwhile, controlling the sampling port valve of the reaction kettle and collecting evaporated ammonia into the distilled water which will be used in a later step of the process. Once the portable Fourier transform infrared gas spectrometer (Matrix-MG, Bruker Co., Ltd. Karlsruhe, Germany) detected that the pH of the outlet gas was acidic, which suggested that there was SO2 and/or SO3 gas overflow, the valve of the sampling port was immediately closed to realize the secondary sulfation of valuable metals in a closed environment. After that, the reaction kettle was naturally cooled to room temperature and the residual ammonia was collected. Finally, the roasted sample was ground to the fineness of <0.074 mm passing 83.6%.
2.2.2 Sulfuric acid leaching and high-value product
The recovery process is shown in Fig. 3. 30 g roasted samples were added in 150 mL of H2SO4 solution (2-14 wt.%). Due to the competitive exchange process of Si, Ca and Mg at low temperature [27], the acid leaching was carried out at 90 °C, 450 r/min for 3 h in a thermostatically- controlled water bath (HJ-6B, Xinrui Instrument Co., Ltd., Jiangsu, China) equipped with a Teflon coated magnetic stirrer. After the acid leaching, the leaching slurry was filtered and yielded a leaching solution rich in metal sulfate and residue, respectively. The effect of the pressurized pyrolysis of AS was measured by the extraction rates of the titanium, magnesium and aluminum. Herein, the extraction rate (η) was calculated by Eq. (1):
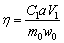 (1)
(1)
where C1, a and V1 were the concentrations of valuable metal in acid leaching solution, the dilution factor and the acid leaching liquid volume, respectively, m0 was the mass of TBBF slag used in this experiment (g), and w0 was the content of titanium, magnesium or aluminum in TBBF slag (mass fraction, %).
The above acid leaching solution was hydrolyzed for 30 min in a boiling state until the steel gray appeared in the solution, and then cooled to room temperature to make the TiO2 crystal nucleus grow up (Eq. (2)). The above precipitate was mixed with 100 mL of distilled water, and subjected to secondary hydrolysis in a boiling state until steel gray appeared again for recrystallization.

Fig. 3 Flow chart of titanium, aluminum and magnesium separation and extraction from Ti-bearing blast furnace slag
The precipitate was filtered and rinsed with distilled water and 2 wt.%, 40 °C H2SO4 solution. Finally, the titanium-rich product was dried overnight at 90 °C, mixed with 0.5 wt.% K2CO3 and calcined at 650 °C for 2 h (Eq. (3)). In terms of TiO2 crystal synthesis, K2CO3 was one of the important parameters to reduce the sintering and agglomeration of TiO2 at high temperature and control the size of grain growth, thereby improving the whiteness and decolorization of TiO2 product.
TiOSO4+nH2O→TiO2·(n-1)H2O↓+H2SO4 (n≥2) (2)
TiO2·SO3·yH2O→TiO2+xSO2↑+yH2O↑ (3)
Before recovering magnesium and aluminum, the main impurity iron must be removed. The above crystallization mother liquor was first adjusted to pH 4 by ammonia at 25 °C, and all Fe3+ ions were precipitated as hydroxides. Then, high value added Al- and Mg-rich products can be obtained through stepwise hydrolysis of the leaching solution. The above crystallization mother liquor reacted with ammonia to increase its pH to be 6, hydrolyzed to fully form Al(OH)3 precipitate and the precipitate was filtered and roasted at 700 °C for 2.5 h to obtain the alumina product. Thus, a completely Fe- and Al-depleted leaching solution was obtained. The crystallization mother liquor further reacted with ammonia to increase its pH≥12.2 for the three-step hydrolysis to obtain Mg(OH)2 precipitate, washing and roasting at 380 °C for 2 h to obtain the magnesium oxide product. AS crystals could be reused through absorbing the residual ammonia and evaporating crystallization.
2.3 Analysis and characterization
The contents of titanium, aluminum and magnesium were measured using inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 7000DV, PerkinElmer Instrument Co., Ltd., Boston, MA, America) at radio frequency (RF) power of 1.35 kW, air flow rate of 12 mL/min, and argon auxiliary flow rate of 0.45 L/min. The phase compositions of TBBF slag and roasted products were analyzed by XRD (Smartlab, Rigaku Corporation, Tokyo, Japan) operated with a Cu Kα radiation source at a wavelength (λ) of 1.5  . Main chemical composition of TBBF slag and high-value products were investigated by XRF (Axios, PANalytical B.V., Almelo, Netherlands) under the conditions of non-attenuating X-ray tube 4 kW, solid state generator power 60 kW, and current 160 mA. The surface morphology of roasted products was performed with scanning electron microscope (SEM, JSM-7001F, JEOL, Tokyo, Japan) at an accelerating voltage of 0.5-30 kV. The relative elemental content of roasted products was analyzed with a combined energy-dispersive X-ray spectrometer (EDS, INCAX-MAX, Oxford, Tokyo, Japan). Thermodynamic calculation of the reaction between TBBF slag and AS was obtained using software HSC (Version 6.0, Outotec Corporation, Outokumpu, Finland).
. Main chemical composition of TBBF slag and high-value products were investigated by XRF (Axios, PANalytical B.V., Almelo, Netherlands) under the conditions of non-attenuating X-ray tube 4 kW, solid state generator power 60 kW, and current 160 mA. The surface morphology of roasted products was performed with scanning electron microscope (SEM, JSM-7001F, JEOL, Tokyo, Japan) at an accelerating voltage of 0.5-30 kV. The relative elemental content of roasted products was analyzed with a combined energy-dispersive X-ray spectrometer (EDS, INCAX-MAX, Oxford, Tokyo, Japan). Thermodynamic calculation of the reaction between TBBF slag and AS was obtained using software HSC (Version 6.0, Outotec Corporation, Outokumpu, Finland).
3 Results and discussion
3.1 Roasting extraction and sulfuric acid leaching
3.1.1 Effect of roasting temperature
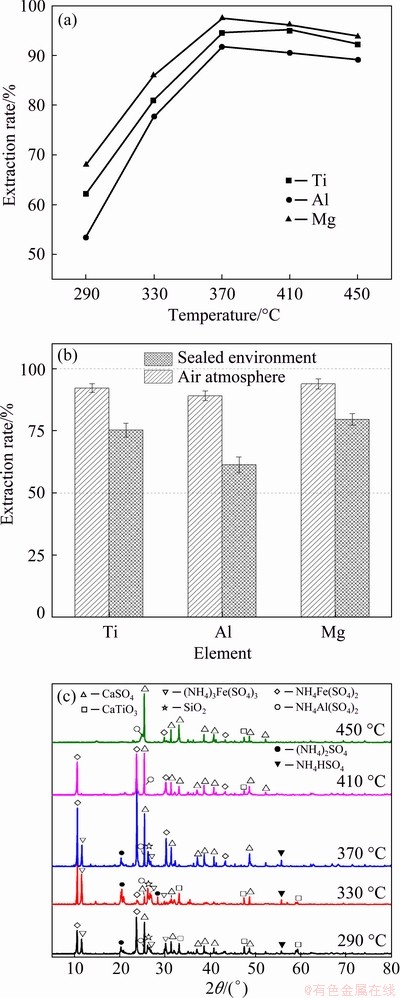
Fig. 4 Extraction rates of titanium, aluminum and magnesium (a), control experiment at different atmospheres (b) and XRD patterns of roasted slags at different temperatures (c)
Figure 4(a) shows the effect of the roasting temperature on extraction of titanium, aluminum and magnesium in the TBBF slag. The extraction rates of titanium, aluminum and magnesium were significantly increased from 62.2% to 94.5%, from 53.5% to 91.9% and from 67.9% to 97.4%, respectively, as the temperature was raised from 290 to 370 °C, suggesting that the soluble sulfates of titanium, aluminum and magnesium remarkably increased under the closed conditions. However, the extraction rates of titanium, aluminum and magnesium at a temperature of 450 °C showed a slightly decline and reached 92.2%, 89.1% and 93.9%, respectively. It might be because the presence of water-insoluble and high-viscosity substances (such as CaSO4 and SiO2) in the reaction system adversely affected the transformation of TBBF slag, hindering the melting of metal and lowering the reaction rate at high temperatures [28]. Therefore, 370 °C was considered optimum.
As a comparison, a control experiment was conducted in the muffle furnace under the air atmosphere with an AS-to-slag mass ratio of 3:1 at 370 °C for 90 min, which was the same as the specific conditions of the sealed environment, and the only difference was whether it was sealed. Leaching conditions were fixed: dilute H2SO4 concentration of 10 wt.%, liquid-to-solid ratio of 5, leaching temperature of 90 °C, leaching time of 3 h and stirring rate of 450 r/min. The results are summarized in Fig. 4(b). Figure 4(b) presents that the extraction rates of titanium, aluminum and magnesium were measured to be 75.3%, 61.4% and 79.6%, respectively. Clearly, these results indicated that the secondary sulfation of SO2 and/or SO3 could significantly promote the sulfation of titanium, aluminum and magnesium in the sealed environment. Meanwhile, it is worth noting that the pressure generated by the internal gas on the autoclave showed a maximum pressure of 0.21 MPa, which was only about twice of the atmospheric pressure. It revealed that the secondary sulfation of SO2 and/or SO3 was dominant and the low pressure had little effect on the phase transformation of mineral during pressurized pyrolysis process. However, the pressure also led to a larger surface area on the surface of the roasted product, potentially, facilitating the likelihood of stable attachment and product trapping [29]. Thus, the synergistic effect of secondary sulfation and low pressure could significantly promote the sulfation of valuable metals.
Figure 4(c) presents that XRD patterns of the roasted slag at different temperatures. The main phases were CaSO4 and the compounded sulfates of aluminum and iron. The diffraction peaks at 2θ of 33.06° and 47.53° were attributed to CaTiO3 at 290 and 330 °C, respectively. When the temperature reached 370 °C, the diffraction peak of CaTiO3 disappeared, indicating that CaTiO3 had completely transformed into its sulfate. The elevating conversion of CaTiO3 from 290 to 370 °C was also consistent with the high extraction rate of titanium in Fig. 4(a). Meanwhile, when the temperature was below 370 °C, the diffraction peaks of (NH4)2SO4 and NH4HSO4 occurred at 2θ of 20.86° and 55.74°, respectively, suggesting that the low temperature could not completely realize the pressure pyrolysis of ammonium sulfate and convert the valuable metals into the corresponding sulfate. The diffraction peaks of the sulphates containing titanium and magnesium were not observed, which may be due to their low crystallinity [30].
3.1.2 Effect of mass ratio of AS to slag
Figure 5(a) shows that the mass ratio of AS to slag had a great influence on the extraction rate of titanium, aluminum and magnesium from the TBBF slag. When the mass ratio of AS to slag was increased from 0.5:1 to 3:1, the extraction rates of titanium, aluminum and magnesium were rapidly increased from 53.9% to 94.5%, from 45.5% to 91.9% and from 60.5% to 97.4%, respectively, indicating that valuable metals had fully reacted to form their sulfates. When the mass ratio was further raised, the extraction rates of titanium, aluminum and magnesium were almost leveled off with their equilibrium values. Thus, taking the cost of AS and the extraction efficiency into consideration, the optimum mass ratio of AS to slag was 3:1.
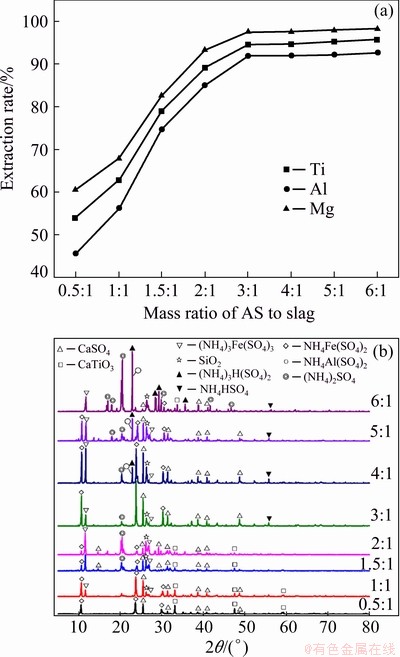
Fig. 5 Extraction rates of titanium, aluminum and magnesium (a) and XRD patterns of roasted slags (b) at different mass ratios of AS to slag
Figure 5(b) shows the XRD patterns of the roasted slags at different mass ratios of As to slag. As shown in Fig. 5(b), only the presence of little AS in TBBF slag could significantly promote the sulfation of valuable metals. Obviously, the phase constituents of roasted products became more complicated with the mass ratio of AS to slag increasing. The diffraction peaks at 2θ of 33.12° and 47.56° were assigned to CaTiO3 from the mass ratio of AS to slag of 0.5:1 to 2:1, respectively, indicating that CaTiO3 was not completely converted at a low mass ratio of AS to slag. Iron and aluminum in the roasting process were well converted into the corresponding phases of NH4Fe(SO4)2, (NH4)3Fe(SO4)3 and NH4Al(SO4)2. When the mass ratio of AS to slag was larger than 3:1, (NH4)2SO4, NH4HSO4 and (NH4)3H(SO4)2 appeared in the roasted sample, suggesting that (NH4)2SO4 was surplus. The analysis also revealed that a suitable mass ratio could significantly promote to recover titanium, aluminum and magnesium from the TBBF slag.
3.1.3 Effect of roasting time
Figure 6 presents that the effects of roasting time on the extraction rates of titanium, aluminum and magnesium in TBBF slag. With the increase of roasting time, the extraction rates of titanium, aluminum and magnesium rapidly rose and reached a plateau within 90 min, i.e., 94.5%, 91.9% and 97.4%, respectively, which were much higher than the corresponding industrial extraction rate [31]. Further increasing the time, the extraction rates of titanium and magnesium slowly decreased to 92.8% and 95.4%, respectively, while the extraction rate of aluminum sharply dropped to 87.8%, which might be due to the more rapid reaction of diopside and perovskite minerals with AS than the reaction of magnesium-aluminium spinel in the early stage. In addition, in terms of thermodynamics, titanyl sulfate (TiOSO4) is unstable to heat [32,33]. Thus, with the increase of time, the water-soluble substance TiOSO4 began to decompose into TiO2 and re-encapsulate by gangue minerals, making it difficult to increase the extraction rate of titanium. Hence, the roasting time of 90 min was chosen to be the optimal parameter.
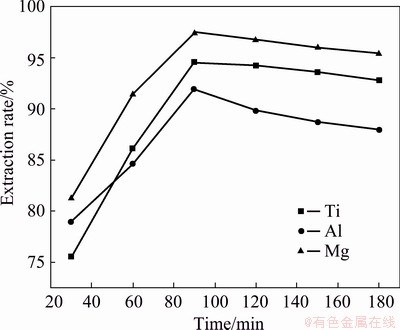
Fig. 6 Extraction rates of titanium, aluminum and magnesium at different roasting time
3.2 Roasted product phase analysis and exploration of leaching conditions
The morphology and component of the roasted product under the optimum conditions were presented in Fig. 7. Figure 7 shows that the roasted sample under optimal conditions was composed of a large amount of porous needle-like crystals which were very irregular. Combined with the EDS analysis, it could be determined that the needle-like particles were NH4Al(SO4)2, CaSO4, TiOSO4 (Points 1 and 3) and a trace of (NH4)3Fe(SO4)3, NH4Fe(SO4)2 and SiO2 (Point 2), indicating that AS greatly promoted the conversion of valuable metals titanium, aluminum and magnesium in TBBF slag to form metal sulfates, which was consistent with the XRD patterns of the roasted sample in Fig. 4(c).
Figure 8 shows that the dosage of H2SO4 was the main parameter affecting the extraction of the titanium, aluminum and magnesium. As shown in Fig. 8, when the dosage of H2SO4 was increased from 2 to 10 wt.%, the extraction rates of titanium, aluminum and magnesium were rapidly increased to 94.5%, 91.9% and 97.4%, respectively, which would greatly dissolve the soluble sulfate inside the mineral by dilute H2SO4 solution. However, when the dosage of H2SO4 was further raised up to 14 wt.%, the extraction rates of titanium, aluminum and magnesium only slightly increased to 95.3%, 92.8% and 98.6%, respectively, suggesting that the titanium, aluminum and magnesium in the raw ore were almost fully dissolved into the leaching solution. Additionally, with the increase of SO2- 4 concentration, the number of coordination sites with Ti4+ increased, which would reduce the active sites of dehydration oxygen bond on Ti4+ and thus inhibited the hydrolysis process [34]. Therefore, considering the cost of materials and the corrosion of equipment by concentrated H2SO4, the dosage of 10 wt.% H2SO4 was optimum.
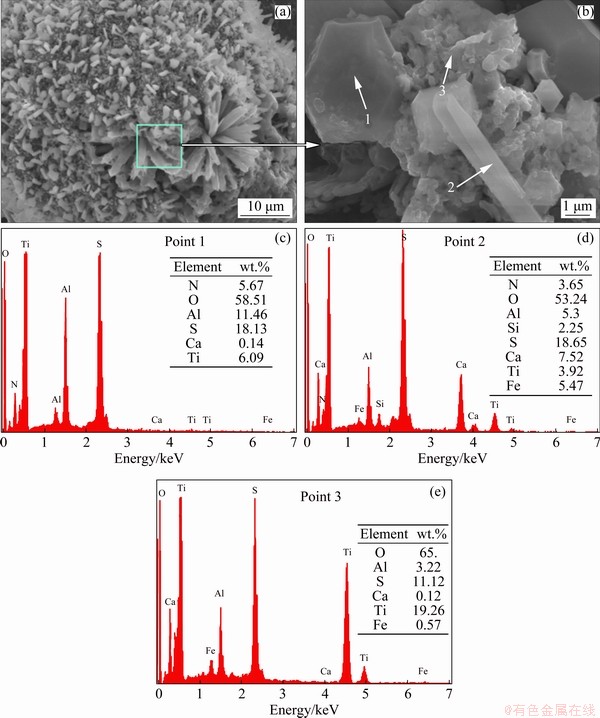
Fig. 7 SEM-EDS analysis results of roasted product

Fig. 8 Extraction rates of titanium, aluminum and magnesium at different dosages of H2SO4
3.3 Preparation of high-value products from leaching solution
Owing to the large difference in hydrolysis pH values of Ti, Mg and Al, selective precipitation for the recovery of Ti, Mg and Al was possible. The formation of titanium-rich product could be achieved by recrystallizing of acid leaching solution in the boiling state (pH≤1), which could remove most of impurities with similar solubility to titanium (such as Fe and Mg). The precipitate was washed alternately with 2 wt.%, 40 °C H2SO4 solution and distilled water to obtain titanium-rich product. Table 2 displays that the chemical compositions of the three products rich in titanium, magnesium and aluminum. The mass fractions of TiO2 and volatile matter were 94.13% and 0.75%, and the whiteness was 95.14, which accorded with the national standard of A2 type TiO2 pigment (Chinese standard, GB 1706—2006).
Subsequently, high value added Mg and Al-rich products could be obtained through adjusting pH and stepwise precipitation of the leaching solution, as described in Section 2.2.2. The concentrations of Mn2+, Fe3+, Al3+, Mg2+ ions were fitted when forming hydroxides at different pH, as shown in Fig. 9 [35]. Figure 9 shows that lg[Me]-pH diagram of Me(OH)n solubility (Me and n stands for metal species and valence, respectively). It can be clearly seen that the above crystallization mother liquor was adjusted to pH 4 by ammonia, and all of Fe3+ ions were precipitated in the form of hydroxide. Then, the Fe(OH)3 precipitate was filtered and separated. When the solution was further adjusted to pH 6, the Al(OH)3 precipitate could be filtered, washed and roasted to obtain alumina products. Table 2 shows that the product contained large quantities of Al2O3 (98.5 wt.%) and a trace of impurities (such as Mg, Ti and Fe), which accorded with the national standard of AO-2 type alumina (Chinese standard, GB 24487—2009).

Fig. 9 lg[Me]-pH diagram of Me(OH)n solubility at 25 °C
The completely Ti- and Al-depleted mother liquor further reacted with ammonia to increase its pH≥12.2 for the three-step hydrolysis to obtain Mg(OH)2 precipitate, washing and roasting at 380 °C for 2 h to obtain the magnesium oxide product. It is noted from Fig. 9 that manganese also enters the Mg(OH)2 precipitate in the form of its hydroxide. However, Table 2 shows that the content of MnO is only 0.93% and the content of MgO is 93.6%, which has reached the national standard of a first-class magnesium oxide (Chinese standard, GB 2573—2012).
3.4 Proposed mechanism for thermal decomposition of TBBF slag with AS
The thermal decomposition mechanism of ammonium sulfate in Fig. 1 shows that AS can produce NH4HSO4, (NH4)3H(SO4)2, NH2SO3H and (NH4)2S2O7 at 370 °C, suggesting that the above sulfates can simultaneously exist. The reactions of the above five sulfates with CaTiO3 and thermodynamic diagrams are shown in Eqs. (4)-(8) (see Table 3) and Fig. 10(a), respectively. Obviously, the Gibbs free energies of the intermediate NH4HSO4, NH2SO3H and (NH4)2S2O7 with perovskite were far below zero, indicating that the reactions could be carried out spontaneously at a normal pressure. For the reaction of (NH4)2SO4 and (NH4)3H(SO4)2 with perovskites, the temperatures corresponding to the Gibbs free energy of zero are 402 and 95 °C, respectively, suggesting that the intermediate (NH4)3H(SO4)2 from thermal decomposition of AS can spontaneously proceed with perovskite under optimal conditions. Thus, the sulfation of metals was not only in favor of the precipitation and growth of the sulfate crystal phase, but also reduce the viscosity of slag [36,37].
Table 2 Chemical compositions of obtained products (wt.%)

The reactions of the above five sulfates with MgAl2O4 and thermodynamic diagrams are shown in Eqs. (9)-(13) and Fig. 10(b), respectively. It could be seen from Fig. 9(b) that the reaction of AS with MgAl2O4 was not spontaneous. However, the other four intermediates produced by AS could spontaneously digest the TBBF slag at 370 °C, which would be accelerated to sulfate under the influence of SO2 and SO3. For the reaction of CaMg(SiO3)2 (Eqs. (14)-(18)) and Fig. 10(c), the five sulfates could react spontaneously under optimal roasting temperatures. In addition, thermodynamic calculation with HSC 6.0 software indicates that Reaction (15) (ΔG=-60.57 kJ/mol) is easier than Reaction (5) (ΔG=-22.91 kJ/mol) and Reaction (11) (ΔG=-52.36 kJ/mol) at 370 °C, which explains why the extraction rate of magnesium is always higher than that of titanium and aluminum, as shown in Figs. 4(a), 5(a) and 6, respectively.
Table 3 Reaction equations of ammonium sulfate and its intermediate products with perovskite, spinel and diopside


Fig. 10 Relationship between Gibbs free energy changes of perovskite reactions (4)-(8) (a), magnesium aluminium spinel reactions (9)-(13) (b) and diopside reactions (14)-(18) (c) and temperature
4 Conclusions
(1) When the TBBF slag was roasted with recyclable AS with a mass ratio 3:1 of AS to TBBF slag at 370 °C for 90 min followed by leaching in a 10 wt.% H2SO4 at 90 °C and 450 r/min for 3 h, the extraction rates of titanium, aluminum and magnesium reached 94.5%, 91.9% and 97.4%, respectively. Meanwhile, the result of a control experiment indicated that the secondary sulfation of SO2 and/or SO3 could significantly promote the sulfation of titanium, aluminum and magnesium in the sealed environment.
(2) The acid leaching solution was subjected to twice hydrolysis in a boiling state to make the content of TiO2 in the titanium product reach 94.1%. The crystallization mother liquor further reacted with ammonia to increase its pH to 6 and ≥12.2, respectively, and the contents of alumina and magnesia obtained reached 98.5% and 93.6%, respectively.
(3) The XRD and EDS analysis proved that main phases of the roasted sample under optimal conditions were NH4Al(SO4)2, CaSO4 and TiOSO4, indicating that AS greatly promoted the conversion of valuable metals in TBBF slag to form corresponding metal sulfate. The thermodynamic analysis showed that the main mineral of perovskite, spinel and diopside in raw ore could spontaneously proceed with the intermediate produced by AS under optimal conditions.
References
[1] LEI Yun, SUN Lu-en, MA Wen-hui, MA Xiao-dong, WU Ji-jun, LI Shao-yuan, MORITA K. An approach to employ titanium-bearing blast-furnace slag to prepare Ti and Al-Si alloys [J]. Journal of Alloys and Compounds, 2018, 769: 983-990.
[2] SUI LI-li, ZHAI Yu-chun. Reaction kinetics of roasting high-titanium slag with concentrated sulfuric acid [J]. Transactions of Nonferrous Metals Society of China, 2014, 14(3): 848-853.
[3] LEI Yun, WANG Chao, MA Wen-hui, WU Ji-jun, WEI Kui-xian, LI Shao-yuan, LV Guo-qiang, MORITA K. A novel approach to prepare high-purity Si and Si/TiSi2 materials simultaneously using Ti-bearing blast furnace slag [J]. Journal of Alloys and Compounds, 2019, 798: 333-341.
[4] ZHEN Yu-lan, ZHANG Guo-hua, CHOU Kuo-chih. Carbothermic reduction of titanium-bearing blast furnace slag [J]. High Temperature Materials and Processes, 2014, 35: 309-319.
[5] LEBOZEC N, THIERRY D, PERSSON D, RIENER C K, LUJENEDER G. Influence of microstructure of zinc- aluminium-magnesium alloy coated steel on the corrosion behavior in outdoor marine atmosphere [J]. Surface and Coatings Technology, 2019, 374: 897-909.
[6] HAN J K, LEE H J, JIANG J I, KAWASAKI M, LANGDON T G. Micro-mechanical and tribological properties of aluminum-magnesium nanocomposites processed by high-pressure torsion [J]. Materials Science and Engineering A, 2017, 684: 318-327.
[7] BELSKAYA O B, LEONTEVA N N, ZAIKOVSKII V I, KAZAKOV M O, LIKHOLOBOV V A. Synthesis of layered magnesium-aluminum hydroxide on the γ-Al2O3 surface for modifying the properties of supported platinum catalysts [J]. Catalysis Today, 2019, 334: 249-257.
[8] WU Min-zhi, LU Hui-hong, LIU Min-chao, ZHANG Zhen-li, WU Xing-rong, LIU Wei-ming, WANG Ping, LI Liao-sha. Direct extraction of perovskite CaTiO3 via efficient dissociation of silicates from synthetic Ti-bearing blast furnace slag [J]. Hydrometallurgy, 2017, 167: 8-15.
[9] YANG Feng-lin, HLVACEK V. Recycling titanium from Ti-waste by a low-temperature extraction process [J]. AlChE Journal, 2000, 46: 2499-2503.
[10] AMANO F. Hydrogen reduced rutile titanium dioxide photocatalyst, titanium dioxide [M]. Tokyo: IntechOpen, 2017.
[11] ZHEN Yu-lan, ZHANG Guo-hua, CHOU Kuo-chih. Mechanism and kinetics of the carbothermic reduction of titanium-bearing blast furnace slag [J]. Metallurgical Research & Technology, 2016, 113: 507.
[12] ADIPURI A, LI Yan-li, ZHANG Guang-qing, OSTROCSKI O. Chlorination of reduced ilmenite concentrates and synthetic rutile [J]. International Journal of Mineral Processing, 2011, 100: 166-171.
[13] JIANG Tao, XUE Xiang-xin, DUAN Pei-ning, LIU Xin, ZHANG Shu-hui, LIU Ran. Carbothermal reduction- nitridation of titania-bearing blast furnace slag [J]. Ceramics International, 2008, 34: 1643-1651.
[14] ZHANG Guo-hua, GOU Hai-peng, WU Ke-han, CHOU Kuo-chih. Carbothermic reduction of Panzhihua ilmenite in vacuum [J]. Vacuum, 2017, 143: 199-208.
[15] FAGHIHISANI M A, YAMAGUCHI A. Oxidation kinetics of MgO-C refractory bricks [J]. Ceramics International, 2002, 28: 835-839.
[16] ZHENG Fu-qiang, GUO Yu-feng, QIU Guan-zhou, CHEN Feng, WANG Shuai, SUI Yu-lei, JIANG Tao, YANG Ling-zhi. A novel process for preparation of titanium dioxide from Ti-bearing electric furnace slag: NH4HF2-HF leaching and hydrolyzing process [J]. Journal of Hazardous Materials, 2018, 344: 490-498.
[17] CHIANG B C, WEY M Y, YEH C L. Control of acid gases using a fluidized bed adsorber [J]. Journal of Hazardous Materials, 2003, 101: 259-272.
[18] NDUAGU E I, HIGHFIELD J, CHEN J, ZEVENHOVEN R. Mechanisms of serpentine–ammonium sulfate reactions: Towards higher efficiencies in flux recovery and Mg extraction for CO2 mineral sequestration [J]. RSC Advances, 2014, 4: 64494-64505.
[19] KONKOLY T I. DSC studies of binary inorganic ammonium compound systems [J]. Journal of Thermal Analysis, 1983, 27: 275-286.
[20] DIOSA J E, FERNANDEZ M E, VARGAS R A, TORIJANO E, MELLANDER B E. On the high-temperature phase transitions of NH4HSO4 [J]. Physica Status Solidi A, 2005, 202: 1891-1895.
[21] HALSTEAD W D. Thermal decomposition of ammonium dulphate [J]. Journal of Applied Chemistry, 1970, 20: 129-132.
[22] MOHAMED S, MERWE E M, ALTERMANN W, DOUCET F. Process development for elemental recovery from PGM tailings by thermochemical treatment: Preliminary major element extraction studies using ammonium sulphate as extracting agent [J]. Waste Management, 2016, 50: 334-345.
[23] ERDEY L, GAL S, LIPTAYI G. Thermoanalytical properties of analytical-grade reagents: Ammonium salts [J]. Talanta, 1964, 11: 913-940.
[24] ZHANG Guo-quan, LUO Dong-mei, DENG Chen-hui, LV Li, LIANG Bin, LI Chun. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process [J]. Journal of Alloys and Compounds, 2018, 742: 504-511.
[25] ZHANG Y. Recovery of titanium from titanium bearing blast furnace slag by sulphate melting [J]. Canadian Metallurgical Quarterly, 2004, 53: 440-443.
[26] WANG Lin, LIU Wei-zao, HU Jing-peng, LIU Qiang, YUE Hai-rong, LIANG Bin, ZHANG Guo-quan, LUO Dong-mei, XIE He-ping, LI Chun. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3 [J]. Chinese Journal of Chemical Engineering, 2018, 26: 583-592.
[27] HIGHFILED J, LIM H Q, FAGERLUND J, ZEVENHOVEN R. Activation of serpentine for CO2 mineralization by flux extraction of soluble magnesium salts using ammonium sulfate [J]. RSC Advances, 2012, 2: 6535-6541.
[28] PICHACANT A, PROVOST E, FURST W, FURST W, HOCHEPIED J F. Determination of the temperature dependence of titanium (IV) hydrolysis and complexation constants in aqueous sulfuric or chlorhydric solutions [J]. The Journal of Chemical Thermodynamics, 2019, 131: 184-191.
[29] BAKHSHIAN S, HOSSEINI S A. Pore-scale analysis of supercritical CO2–brine immiscible displacement under fractional–wettability conditions [J]. Advances in Water Resources, 2019, 126: 96-107.
[30] WANG Ming-yu, LOU Tai-ping, ZHANG Li, SUI Zhi-tong. Separation of iron droplets from titania bearing slag [J]. Journal of Iron and Steel Research International, 2008, 15: 45-48.
[31] XIE Keng, ZHAO Jun-mei, YANG Liang-rong, YU Pin-hua, LIU Hui-zhou. Investigation of three-liquid-phase extraction systems for the separation of Ti (IV), Fe (III) and Mg (II)) [J]. Separation and Purification Technology, 2010, 76: 191-197.
[32] JOHNSSON M, PETTERSSON P, NYGREN M. Thermal decomposition of fibrous TiOSO4·2H2O to TiO2 [J]. Thermochimica Acta, 1997, 298: 47-54.
[33] KOLEN'KO Y V, BURUKHIN A A, CHURAGULOV B R, OLEYNIKOV N N. Synthesis of nanocrystalline TiO2 powders from aqueous TiOSO4 solutions under hydrothermal conditions [J]. Materials Letters, 2003, 57: 1124-1129.
[34] LEVIN I, KRAYZMAN V, VANDERAH T A, TOMCZYK M, WU H, TUCKER M G, PLAYFORDD H Y, WOICIKA J C, DENNISA C L, VILARINHOB P M. Oxygen-storage behavior and local structure in Ti-substituted Y MnO3 [J]. Journal of Solid State Chemistry, 2017, 246: 29-41.
[35] LI Fa-mei. Analytical chemistry [M]. 6th ed. Beijing: People’s Health Press, 2007. (in Chinese)
[36] ALJERF L, ALMASRI N. Excellent crystal coloration and an extraordinary improvements of developing synthetic quartz single crystals growth and defects [J]. Archive of Organic and Inorganic Chemical Sciences, 2018, 3: 358-365.
[37] ZHANG L, ZHANG L N, WANG M Y, LOU T P, SUI Z T, JANG J S. Effect of perovskite phase precipitation on viscosity of Ti-bearing blast furnace slag under the dynamic oxidation condition [J]. Journal of Non-crystalline Solids, 2006, 352: 123-129.
边振忠1,冯雅丽1,李浩然2
1. 北京科技大学 土木与资源工程学院,北京 100083;
2. 中国科学院 过程工程研究所 生化工程国家重点实验室,北京 100190
摘 要:提出一种硫酸铵(AS)加压热解-酸浸新工艺,从含钛高炉渣中(TBBF)分段回收有价金属。结果表明,当在铵矿比3:1、370 °C的条件下热解90 min时,钛、铝和镁的提取率分别为94.5%、91.9%和97.4%。酸浸液在沸腾状态下重结晶除杂可获得TiO2含量为94.1%的钛产品。上述结晶母液分别调节pH=6和pH≥12.2,可获得合格的氧化铝和氧化镁产品。XRD和SEM-EDS分析表明,焙烧样品中主要物相为NH4AlSO4、CaSO4 和TiOSO4。热力学分析显示,在最优条件下,原矿中钙钛矿、镁铝尖晶石和透辉石能与AS产生的中间产物自发反应。
关键词:含钛高炉渣;硫酸铵;加压热解;高值化产品
(Edited by Xiang-qun LI)
Foundation item: Project (DY135-B2-15) supported by China Ocean Mineral Resources R&D Association; Project (2015ZX07205-003) supported by Major Science and Technology Program for Water Pollution Control and Treatment, China; Projects (21176242, 21176026) supported by the National Natural Science Foundation of China
Corresponding author: Ya-li FENG, Tel: +86-13910839080, E-mail: ylfeng126@126.com;
Hao-ran LI, Tel: +86-13701399765, E-mail: hrli@ipe.ac.cn
DOI: 10.1016/S1003-6326(20)65425-5

