空气氧化法制备Fe3O4的反应机理
杨喜云,龚竹青,彭长宏,陈白珍,徐 徽,石西昌
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:采用空气氧化法制备Fe3O4,分析反应过程中pH值随时间变化曲线和Fe的形态变化规律;用XRD和SEM检测反应各个阶段沉淀物的物相组成和形貌变化。研究结果表明:在中性和酸性介质中,Fe3O4的生成分3个阶段:Fe(OH)2悬浮液氧化为绿锈,绿锈转变为球形Fe3O4,Fe3O4氧化为针形FeOOH;氧化反应发生在液相,随着氧化的进行,pH值和液相Fe2+浓度发生改变,Fe3O4粒度不断增大;制备Fe3O4时,应控制pH值大于5.4,固相Fe(Ⅲ)与Fe(Ⅱ)的摩尔比小于1.8。
关键词:空气氧化法;Fe3O4;反应机理
中图分类号:TQ 031.7 文献标识码:A 文章编号:1672-7207(2008)03-0454-05
Formation mechanism of magnetite prepared by air oxidation
YANG Xi-yun, GONG Zhu-qing, PENG Chang-hong, CHEN Bai-zhen, XU Hui, SHI Xi-chang
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Magnetite was prepared by air oxidation. Relationship between pH value and time was analyzed and the state change of iron was recorded during the formation process. Phase composition and morphology of the precipitates were detected with XRD and SEM at several stages of oxidation, respectively. The results show that three stages are observed during Fe3O4 formation in neutral and acidic media, the process is that Fe(OH)2 suspension is oxidized to green rust followed by converting to spherical magnetite particles, then some magnetite particles gradually oxidize to acicular FeOOH. The oxidation occurs in the aqueous solution. As oxidation continues, pH value and Fe(Ⅱ) concentration in supernatant liquid change with time, and Fe3O4 particle size increases. To get Fe3O4 with good quality, pH value should be greater than 5.4, and the molar ratio of Fe(Ⅲ) to Fe(Ⅱ) in the solid phase should be less than 1.8.
Key words: air oxidation; Fe3O4; formation mechanism
Fe
3O
4具有许多优异的性能,在磁性材料、催化剂、生物化学等传统领域应用广泛
[1-6]。Fe
3O
4磁粉常用的制备方法是中和法和氧化法。采用中和法成本高,产品粒度分布范围宽,磁性能差;采用氧化法所得产品质量高,磁性能好,但氧化反应过程复杂,对产品质量影响大。目前,我国大多数高质量的Fe
3O
4都依靠进口,因此,研究水溶液中Fe
3O
4制备过程,有利于了解Fe
3O
4的生成规律,提高产品性能。人们对氧化法制备Fe
3O
4进行了研究,如M. Misawa等
[7]认为,在室温下,钢被酸腐蚀溶解为Fe
2+,Fe
2+转化为Fe(OH)
2,Fe(OH)
2在空气中氧化为绿锈,绿锈慢速氧化为Fe
3O
4,快速氧化为γ-FeOOH。M. Kiyama
[8]将Fe
3O
4的生成过程描述为:Fe(OH)
2悬浮液的生成;Fe
3+氧化物的生成,如γ-FeOOH等;γ-FeOOH等氧化物缓慢转化为Fe
3O
4,反应中还可以生成α-FeOOH和γ-FeOOH等副产物。C. Domingo等
[9]认为Fe
3O
4的生成过程是Fe(OH)
2的溶解、氧化、成核过程,Fe
3O
4生长在Fe(OH)
2的表面上,Fe(OH)
2的氧化速度随H
+浓度增加而减小。以上研究结果只是研究者根据有限实验结果进行推测得出的,且所研究的体系反应温度低,生成物不要求为纯Fe
3O
4,所以,掌握中间的变化过程,找出关键的影响因素还需进一步研究。本文作者
[10]曾对氧化沉淀法制备Fe
3O
4的动力学进行研究,得到了空气流速对反应过程的影响规律,给出了反应速率方程。在此,通过分析pH值随时间t变化以及Fe的形态变化规律,监测反应过程物相来研究Fe
3O
4生成过程,指出影响Fe
3O
4生成的关键因素及控制方法,以便为制备优质产品提供依据。
1 实 验
1.1 Fe3O4的制备过程
取适量FeSO4溶液置于5口烧瓶中,开启搅拌器,恒定反应温度为80 ℃,用 N2驱走烧瓶中的空气,在N2保护下,1次添加化学计量(NaOH与Fe2+形成Fe(OH)2所需NaOH的量)为85%的碱液(即[Fe]/[OH-]= 1/(2×0.85)=0.588),形成Fe(OH)2悬浮液,20 min后将N2切换成空气,开始记录时间及pH值,初始Fe2+浓度为0.2 mol/L,反应总体积保持为1.5 L。
1.2 分析检测
在反应过程中定时取样分析,每次取悬浮液5 mL,用重铬酸钾法分析Fe(Ⅱ)和总铁浓度。当分析上清液时,每次取悬浮液10 mL,离心过滤,使固液分离,所得滤液称为上清液,用重铬酸钾法分析其中Fe(Ⅱ) 和Fe(Ⅲ)浓度,所得固体用蒸馏水和丙酮洗涤,在氮气保护下干燥,采用日本理学Rigaku D/MAX 2500型X射线衍射仪检测固体物相,采用JEOL公司制造的JSM-6360LV型扫描电镜观察反应过程中固体粒子形貌。
2 结果与讨论
在反应过程中,悬浮液颜色和黏度发生一系列变化:颜色由初始白色变为墨绿色,再转变为黑色;颜色为墨绿色时,黏度达到最大,并且反应条件对反应过程影响较大。
2.1 悬浮液pH值与时间t的关系
反应过程中悬浮液pH值随t的变化曲线如图1所示。从图1可以看出,体系pH值开始下降很快(阶段Ⅰ),然后变化平缓(阶段Ⅱ),最后又急剧下降(阶段Ⅲ)。
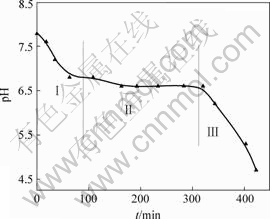
图1 悬浮液pH值与时间t的关系
Fig.1 Relationship between pH value of suspension and oxidation time
在第Ⅰ阶段,起始pH值为7.8,经过90 min 后pH值下降为6.6,经分析此阶段大约有15% Fe(Ⅱ)被氧化为Fe(Ⅲ)。由于反应中只加入85% 的碱液,悬浮液中有游离的Fe(Ⅱ),主要以Fe2+和FeOH+ 2种形式存在,且以FeOH+为主[11]。R. Lin等[12]认为,在第Ⅰ阶段,pH值下降可能是Fe(Ⅱ)发生氧化产生Fe(Ⅲ),Fe(Ⅲ)水解所致,反应式如下:
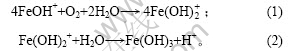
为了判断Fe(Ⅲ)水解是否会导致pH值下降,考查了2 mol/L Fe2(SO4)3溶液对上述反应体系pH值的影响,结果如图2所示。其中,在A→B和E→F阶段通入空气,在B→C和D→E阶段停止通气,在C和D处加入3 mL和2.4 mL 2 mol/L Fe2(SO4)3溶液。在B→C阶段,pH值稍有回升,在C点,由于慢慢加入Fe2(SO4)3溶液,控制加入速度与Fe(Ⅲ)生成速度基本相同,加入Fe(Ⅲ)引起的pH值下降与通气过程的相似,说明Fe(Ⅲ)水解是导致pH值下降的原因。在反应第Ⅱ阶段D点加入Fe(Ⅲ),对pH值的影响很短暂,且基本不影响第Ⅱ阶段的氧化时间。
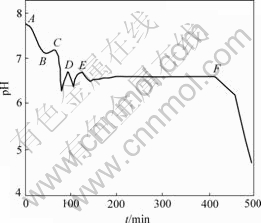
图2 加入2 mol/L Fe2(SO4)3后悬浮液中pH值与时间t的关系
Fig.2 Relationship between pH value of suspension and oxidation time after adding 2 mol/L Fe2(SO4)3
在第Ⅱ阶段,加入1 mol/L NaOH后的pH值与反应时间的关系如图3所示。其中,在A→B,C→D和E→F阶段通入空气,在B和D处分别加入3 mL和1.8 mL 1 mol/L NaOH溶液。由图3可知,加入NaOH后,pH值急剧上升;当通入空气后,pH值又迅速回到加NaOH前的值,说明通空气氧化时H+产生速度快。

图3 加入1 mol/L NaOH后悬浮液pH值与时间t的关系
Fig.3 Relationship between pH value of suspension and oxidation time after adding 1 mol/L NaOH
2.2 悬浮液中Fe的形态变化规律
在不同时间将悬浮液离心沉降,分析Fe的形态变化规律。图4所示为上清液Fe(Ⅱ)浓度随时间的变化曲线,图5所示为悬浮液中Fe(Ⅱ)与∑Fe摩尔比随时间的变化曲线,图6所示为固相Fe(Ⅲ)与Fe(Ⅱ)摩尔比随时间的变化曲线。
从图4可以看出:在第Ⅰ阶段,上清液Fe(Ⅱ)浓度随时间增加而减小;在第Ⅱ阶段,上清液Fe(Ⅱ)浓度随时间增加而增大,当时间超过330 min后,进入第Ⅲ阶段,pH值下降,上清液Fe(Ⅱ)浓度增加,当达到0.032 mol/L时,与氧化前Fe(Ⅱ)理论浓度0.03 mol/L接近时,不再随时间增加。由图1可知,此时体系pH值已低于4.5,所以,Fe(Ⅱ)氧化速度很慢,浓度变化小。R. Lin等[12-13]采用空气氧化法生成γ-FeOOH时,发现Fe(Ⅱ)浓度随时间的变化也有类似情况。
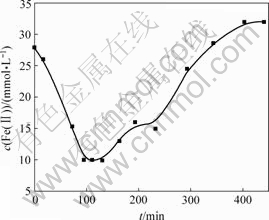
图4 上清液Fe(Ⅱ)浓度与时间t的关系
Fig.4 Relationship between concentration of Fe(Ⅱ) in supernatant liquid and oxidation time
从图5可以看出,悬浮液中Fe(Ⅱ)与∑Fe摩尔比随时间呈直线下降,在反应过程中空气流速是一定的,说明氧化反应速度与反应过程中Fe(Ⅱ)浓度减小、Fe3O4质量增加无关,所以,氧化反应发生在液相。
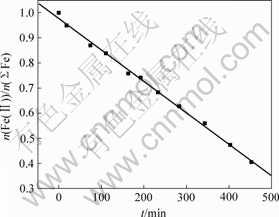
图5 悬浮液中Fe(Ⅱ)与总Fe摩尔比随时间t的变化
Fig.5 Relationship between molar fraction of Fe(Ⅱ) to total Fe and time in suspension
从图6可以看出,在反应后期固相中Fe(Ⅲ)与Fe(Ⅱ)摩尔比上升很快,经实验发现,当Fe(Ⅲ)与Fe(Ⅱ)的摩尔比超过1.8时,产品呈棕色,此时体系pH值为5.4。所以,要制备出高质量的Fe3O4,应严格控制悬浮液pH值大于5.4,Fe(Ⅲ)与Fe(Ⅱ)摩尔比小于1.8。
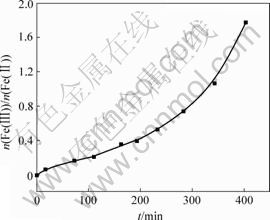
图6 固相Fe(Ⅲ)/Fe(Ⅱ)摩尔比随时间t的变化
Fig.6 Relationship between molar ratio of Fe(Ⅲ) to Fe(Ⅱ) and time in solid phase
2.3 物相和形貌
在反应过程中分别在130,290,403和470 min取样分析,其XRD图谱如图7所示,SEM图谱如图8所示。从图7可以看出,在130 min时,绿锈含量为92.4%,Fe3O4含量为7.6%,此时大约有21% Fe(Ⅱ)被氧化,绿锈分子式可简单表示为[Fe(Ⅱ)4Fe(Ⅲ)- On(OH)9-2n]?SO4;在290 min时,Fe3O4含量为47.9%,绿锈含量为53.1%,大约有44% Fe(Ⅱ)被氧化;在403 min时,全部转化为Fe3O4,有53% Fe(Ⅱ)被氧化,在470 min时,主要成分为Fe3O4,含有极少量FeOOH,是Fe3O4过氧化所致,在整个反应过程中没有检测到Fe(OH)2和Fe(OH)3,与M. Misawa等[7]描述的酸性环境中生成Fe3O4时有Fe(OH)2中间产物的观点不相同。
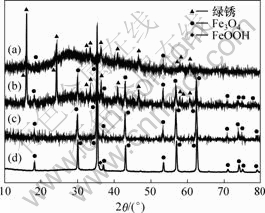
t/min: (a) 130; (b) 290; (c) 403; (d) 470
图7 不同反应时间所得沉淀物的XRD图
Fig.7 XRD patterns of precipitates at different reaction time
从图8可以看出,当反应130 min时,出现大量无定型绿锈颗粒,并有极少量Fe3O4球形颗粒;反应进行到290 min时,出现大量球形颗粒,绿锈形貌由无定型颗粒变为六方密集型颗粒;反应403 min时,全为球形Fe3O4颗粒;反应470 min时,除大量球形颗粒外,还含有少量FeOOH针形颗粒。对比球形颗粒粒径可以看出,随着时间的延长,颗粒不断长大。
2.4 反应机理
根据前面分析可知:在第Ⅰ阶段,绿锈生成,液相pH值降低,Fe(Ⅱ)浓度降低;在第Ⅱ阶段,绿锈转化为Fe3O4,液相pH值基本不变,Fe(Ⅱ)浓度升高;在第Ⅲ阶段,绿锈继续转化为Fe3O4,有少量Fe3O4氧化为FeOOH,液相pH值下降,Fe(Ⅱ)浓度继续升高至氧化前的初始值,整个反应过程氧化反应速度一定。所以,Fe3O4 形成的总反应方程式可以表示为

由式(9)可以看出,绿锈转化为Fe3O4,释放大量Fe(OH)+。所以,在第Ⅱ和第Ⅲ阶段,Fe(Ⅱ)浓度增加,一直达到氧化前的初始值后才保持不变。
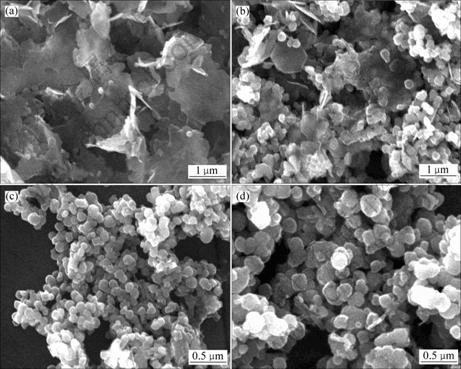
t/min: (a) 130; (b) 290; (c) 403; (d) 470
图8 不同反应时间所得沉淀物的SEM图
Fig.8 SEM images of precipitates at different reaction time
图3所示结果说明,通空气氧化时,H+产生速度快,而由式(9)可以看出,绿锈转化为Fe3O4释放OH-,H+产生速度与OH-释放速度基本相等,所以,在第Ⅱ阶段pH值基本不变;在第Ⅲ阶段,绿锈很少,氧化反应仍继续,氧化产生H+导致pH值下降。
3 结 论
a. 在酸性和中性环境,Fe3O4生成经历3个阶段:第Ⅰ阶段,Fe(OH)2悬浮液氧化为绿锈;第Ⅱ阶段,绿锈转化为Fe3O4;第Ⅲ阶段,绿锈继续转化为Fe3O4,有少量Fe3O4氧化为FeOOH。
b. 氧化反应发生在液相,H+产生速度快,消耗速度慢,Fe(Ⅱ)氧化水解引起pH值下降。
c. 制备高质量的Fe3O4应严格控制pH值大于5.4,固相Fe(Ⅲ)与Fe(Ⅱ)摩尔比小于1.8。
d. 随着氧化反应进行,pH值和液相Fe(Ⅱ)浓度发生改变,无定型颗粒发展为六方密集型颗粒,并转变成球形Fe3O4,其粒度不断增大;时间长会导致过氧化,部分Fe3O4氧化成针形FeOOH颗粒。
参考文献:
[1] Nihira Y. Magnetic oxide powder for toner and magnetic toner: Japan, JP11024305[P]. 1999-01-29.
[2] Tamaura Y, Tabata M. Complete reduction of carbon dioxide to carbon using cation-excess magnetite[J]. Nature, 1990, 346(6281): 255-258.
[3] Shen L, Laibinnis P E, Hatton T A. Bilayer surfactant stabilized magnetic fluids:synthesis and interactions at interfaces[J]. Langmuir, 1999, 15(2): 447-453.
[4] Zhang Y, Kohler N, Zhang M Q. Surface modification of superparamagnetic magnetite nanoparticles and intracellular uptake[J]. Biomaterials, 2002, 23(7): 1553-1561.
[5] Meisen U, Kathrein H. The influence of particle size, shape and particle size distribution on properties of magnetite for the production of toners[J]. Journal of Imaging Science and Technology, 2000, 44(6): 508-513.
[6] Velsen F M, Vos G. High gradient magnetic separation technique for waste treatment[J]. Water Science and Technology, 1991, 24(10): 195-203.
[7] Misawa M, Hashimoto K, Shimodaira S. Mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature[J]. Corrosion Science, 1974, 14(2): 131-149.
[8] Kiyama M. Conditions for the formation of Fe3O4 by the air oxidation of Fe(OH)2 suspensions[J]. Bulletin of the Chemical Society of Japan, 1974, 47(7): 1646-1650.
[9] Domingo C, Rodrigue C R, Blesa M A. Pathways to spinel iron oxides by oxidation of iron(Ⅱ) in basic media[J]. Materials Research Bulletin, 1991, 26(1): 47-55.
[10] YANG Xi-yun, GONG Zhu-qing. Kinetics of Fe3O4 formation by air oxidation[J]. Journal of Central South University of Technology, 2004, 11(2): 152-155.
[11] 丁 明. 中和沉淀法Fe3O4的生成过程研究[J]. 成都大学学报, 1998, 17(2): 16-19.
DING Ming. Study on Fe3O4 formation processing by neutral precipitation[J]. Journal of Chengdu University, 1998, 17(2): 16-19.
[12] Lin R, Spicer R L, Tungate F L, et al. Study of the oxidation of ferrous hydroxide in slightly basic solution to produce γ-FeOOH[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1996, 113(1/2): 79-96.
[13] Srinivasan R, Lin R, Spicer R L, et al. Structural features in the formation of the green rust intermediate and γ-FeOOH[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1996, 113(1/2): 97-105.
收稿日期:2007-09-28;修回日期:2007-11-26
基金项目:湖南省自然科学基金资助项目(08JJ3097);国家高技术发展计划资助项目(2006AA06Z373)
通信作者:杨喜云(1974-),女,湖南邵东人,博士,副教授,从事功能材料方面的研究;电话:0731-8877352;E-mail: yxy7412@mail.csu.edu.cn