文章编号:1004-0609(2012)04-1248-07
熔融NaOH分解橄榄石型硅酸盐的反应机理
徐 敏1, 2,许 茜1,刘日强1,王子睿1,翟玉春1
(1. 东北大学 材料与冶金学院,沈阳 110004;
2. 黑龙江公安警官职业学院 刑事科学技术系,哈尔滨 150025)
摘 要:采用熔融NaOH分解Mg2SiO4 和MgNiSiO4,采用拉曼光谱对碱熔融过程进行在线检测,利用X射线衍射仪和红外光谱仪等分析碱融后的水浸渣,研究硅酸盐在熔融碱中的分解机理。结果表明:NaOH对Mg2SiO4中Mg离子的释放是逐次进行的,其中间产物为Na2MgSiO4;MgNiSiO4在碱熔融分解过程中,Ni离子优先于Mg离子从硅酸盐阵列中释放;镁和镍橄榄石型硅酸盐中的Mg—O和Ni—O最终都可以被Na—O替换,生成Na4SiO4。
关键词:Mg2SiO4;MgNiSiO4;碱熔融;反应机理
中图分类号:TQ170.1 文献标志码:A
Reaction mechanisms of decomposition of magnesium nickel silicate by alkali fusion using NaOH
XU Min1, 2, XU Qian1, LIU Ri-qiang1, WANG Zi-rui1, ZHAI Yu-chun1
(1. School of Materials Science and Metallurgy, Northeastern University, Shenyang 110004 China;
2. Department of Criminal Science and Technology, Heilongjiang Police Officer Vocational College,
Harbin 150025, China)
Abstract: The decomposition of forsterite and magnesium-nickel silicate was undertaken by alkali fusion using NaOH at elevated temperatures. Raman spectra were measured for the reactions between the silicates and sodium hydroxide in-situ during the alkali fusion process. Meanwhile, the water-leaching residue of the fusion products was characterized by X-ray diffractometry and infrared spectroscopy. The results show that the magnesium ions in Mg2SiO4 can be liberated from the silica arrays successively by aggression of sodium hydroxide, giving rise to Na2MgSiO4 as an intermediate; whereas nickel ions seem to be released much easier from the arrays of MgNiSiO4 than magnesium ions, and move out from their array sites prior to magnesium ions. The bindings between Mg—O and Ni—O in their silicates can be substituted by Na—O completely, and Na4SiO4 is one of the final products for the alkali fusion process.
Key words: Mg2SiO4; MgNiSiO4; alkali fusion; reaction mechanism
金属镍由于具有良好的机械强度和延展性[1]、高熔点、高化学稳定性、空气中抗氧化等特征成为现代航空工业、国防工业和人们生活中不可缺少的重要金属之一。随着镍需求量的增加以及世界镍硫化矿资源日益短缺,如何高效率、低成本、无污染地利用镍的氧化矿资源 —— 红土镍矿的重要性日益凸显。红土镍矿资源储量约占全球镍资源的72%,镍红土矿主要有两种类型,一种是褐铁矿型的红土矿,另一种是硅酸盐型的红土矿。硅酸盐型的镍红土矿的主要构成是镁、铁的硅酸盐,镍则以类质同相取代铁或镁存在于硅酸盐中,镍的品位在1%~2%之间[2-5]。
为了从硅酸盐矿物中有效地分离镍元素,需要破坏其稳定的硅酸盐结构,使镍元素可以从硅氧四面体组成的阵列束缚中释放出来。利用熔融碱或高浓度碱液可以提供成键的OH-分解硅酸盐结构,因此,碱处理成为分解硅酸盐型矿物的有效方法之一。目前,尽管人们在碱处理硅酸盐型矿物方面已经开展了许多研究工作,但是研究主要集中在唯像学的范畴,例如对碱处理工艺条件的优化,以及利用宏观动力学研究碱处理过程的动力学方程、确定反应的控制步骤和反应的表观活化能[6-8]。研究碱处理两性金属氧化物矿资 源仍是一个尚需深入的领域,特别是基于碱处理过程中矿相结构的转变、化学键的变化以及无机聚合物网络结构的重构等角度研究硅酸盐碱转化过程和反应机理,将会帮助人们更深刻地理解碱处理硅酸盐型矿物的反应机理,从而实现对碱分解过程的有效控制,以利于后续的浸出过程。这对于高效利用硅酸盐型红土镍矿具有重要意义。
本文作者以溶液沉淀和固相反应相结合的方法合成Mg2SiO4和MgNiSiO4,利用X射线衍射仪、红外光谱仪等对碱熔融后的水浸渣进行测试,同时对熔融碱处理Mg2SiO4过程进行拉曼光谱分析,研究Mg2SiO4和MgNiSiO4在熔融NaOH中的反应机理和转化行为,为碱熔融法高效处理低品位镍红土矿提供理论依据。
1 实验
实验所用NaOH、Na2SiO3、MgCl2、MgSO4、MgO和NiO均为分析纯。水为去离子水。采用水溶液沉淀法合成MgSiO3;利用合成的MgSiO3与MgO和NiO分别在1 200 ℃,10 h和1 100 ℃,5 h固相反应制备Mg2SiO4和 MgNiSiO4。
熔融NaOH分解Mg2SiO4和 MgNiSiO4反应在镍坩埚中进行,熔融碱处理采用两种实验方法,其一是将一定量的固体氢氧化钠加入镍坩埚中,加热至设定温度,待氢氧化钠完全溶化后,投入确定反应剂量的Mg2SiO4或 MgNiSiO4,并开始对反应计时(称为投料式);其二是将Mg2SiO4与给定反应剂量的NaOH提前混合后,一起加热至设定温度后开始计时(称为预先混料式)。反应经过一段时间后,将反应体系降至室温并加水浸出,通过离心分离得到含可溶性硅酸盐的浸出液和碱熔水浸渣。
水浸渣分别采用日本理学Rigaku X射线仪和Nicolet 380 FT-IR型智能傅立叶红外光谱仪分析;碱熔融过程利用Lab RAM HR-800型拉曼光谱仪进行在线分析。
2 结果与讨论
2.1 Mg2SiO4和MgNiSiO4合成与表征
图1所示为本研究合成Mg2SiO4和MgNiSiO4产物的XRD谱,橄榄石相硅酸镁Mg2SiO4(JCPDS file 71—1972) 和硅酸镁镍MgNiSiO4(JCPDS file 203017)的标准XRD谱也示于图1中。
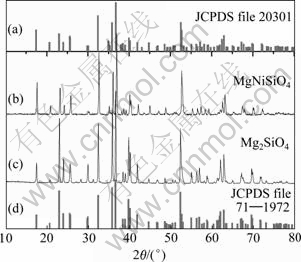
图1 合成镁镍橄榄石和镁橄榄石的XRD谱
Fig. 1 XRD patterns of olivines prepared: (a) MgNiSiO4 standard JCP data; (b) Mg2SiO4 prepared; (c) MgNiSiO4 prepared; (d) Forsterite standard JCP data
由图1可知:橄榄石相的正硅酸镁和正硅酸镁镍XRD的标准谱非常相似,但是,橄榄石相的正硅酸镁镍XRD衍射峰整体向高角度方向略有移动。合成的正硅酸镁镍和正硅酸镁与其橄榄石相的XRD吻合很好;两者相比,合成的正硅酸镁镍XRD衍射峰较正硅酸镁的衍射峰也整体向高角度方向移动。合成的MgNiSiO4可以看成是Mg2SiO4异质同相体。由于Ni2+和Mg2+半径相近,Ni2+可以部分取代Mg2+,占据了Mg2SiO4晶体中Mg的晶格点阵位置,导致样品的晶胞参数和晶胞V值改变(见表1)、以及XRD衍射峰向高角度移动。在镁-镍橄榄石中,Mg2+和Ni2+的分布部分有序。
图2所示为合成的正硅酸镁和正硅酸镁镍扫描SEM像,并对SEM像中标出点的成分进行EDS能谱分析。对于图2中标出点①处,Mg、Si、O的摩尔比近似为1.9:1.0:3.8;标出点②处,Mg、Ni、Si、O的摩尔比为1.0:0.9:1.0:4.3。结合XRD物相分析结果可以确定本研究依次合成了橄榄石型正硅酸镁和正硅酸镁镍。
表1 Mg2SiO4和MgNiSiO4的晶胞参数比较
Table 1 Unit-cell parameters of Mg2SiO4 and MgNiSiO4 prepared in this study
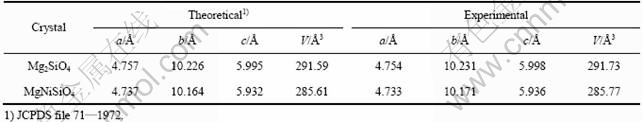

图2 合成的正硅酸镁和正硅酸镁镍的SEM像
Fig. 2 SEM images of magnesium silicate(a) and magnesium-nickel silicate(b)
2.2 碱熔后水浸渣的XRD物相分析
图3(a)所示为在400 ℃、15 min条件下Mg2SiO4碱熔融水浸渣的XRD谱。从XRD谱可以得到,对于不同碱矿比的体系经过15 min碱熔融反应,Mg2SiO4与熔融NaOH的反应产物中都可以发现Na2MgSiO4中间产物生成。反应可以表示为
Mg2SiO4+NaOH=Na2MgSiO4+Mg(OH)2 (1)
虽然在XRD谱中未见Mg(OH)2, 仅出现MgO衍射峰,这是由于水浸渣在煅烧后Mg(OH)2分解成MgO;但是,对未经煅烧的水浸渣红外光谱分析(见图4) 明显可见Mg—OH键振动峰和Mg(OH)2晶格的特征谱。对碱熔后水浸渣的XRD分析还可以看到增加碱量有助于Mg2SiO4转化成Mg(OH)2。
当采用预先混料方式进行400 ℃、15 min碱熔融反应后, Mg2SiO4-NaOH体系的反应产物经过水浸后煅烧,其XRD谱见图3(b)。结果显示水浸渣中仅含Mg(OH)2的分解产物MgO,因此,可以断定几乎所有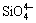 都与Na+结合,生成可溶性的硅酸钠进入水溶液相。这是由于采用预先混料的实验方式,Mg2SiO4与NaOH反应时间远超过15 min,表明通过延长碱熔融的反应时间可以实现Mg2SiO4完全转化成可溶性硅 酸钠。
都与Na+结合,生成可溶性的硅酸钠进入水溶液相。这是由于采用预先混料的实验方式,Mg2SiO4与NaOH反应时间远超过15 min,表明通过延长碱熔融的反应时间可以实现Mg2SiO4完全转化成可溶性硅 酸钠。
图4所示为MgNiSiO4在400 ℃反应条件下(碱与原料摩尔比为10:1)碱熔融15 min后渣的XRD谱。由图4可以看出,MgNiSiO4碱熔融反应后渣相的主要成分为Na2MgSiO4、NiO和少量MgNiSiO4。由于Na2MgSiO4与Na2NiSiO4是异质同相,所以,MgNiSiO4的碱熔融产物中也可能含有Na2NiSiO4。但是,其XRD谱中显示的氧化物的衍射峰明显偏向于NiO的衍射峰,说明MgNiSiO4碱熔融反应过程中,Ni+应优先于Mg2+与NaOH中Na+交换,生成Ni(OH)2,经煅烧后得到NiO。
2.3 碱熔融反应后的水浸渣红外光谱分析
图5所示为不同碱量时于400 ℃、15 min碱熔融后水浸渣的红外光谱,以及室温下Mg2SiO4和Mg(OH)2的红外光谱。其中,NaOH与Mg2SiO4质量比为30:1水浸渣经过600 ℃煅烧,其余水浸渣经室温干燥。水浸渣的红外光谱在3 424 cm-1附近的较宽吸收峰是浸出渣中所含吸附水的O—H伸缩振动[9];在 3 700 cm-1处的吸收峰应为Mg—OH的伸缩振动,因为对于经过600 ℃煅烧后的浸出渣未见该吸收峰,可能的原因是Mg(OH)2经过煅烧后大部分分解成MgO。这一结果与WU等[10]认为在3 700 cm-1和3 424 cm-1附近吸收峰皆为O—H伸缩振动略有差别。1 651 cm-1附近和1 443 cm-1的吸收峰应属于Mg—OH的弯曲振动[11];在1 000~800 cm-1波数范围内出现的吸收峰为SiO4的伸缩振动峰[12],对于Mg2SiO4在994、898和839 cm-1处依次出现明显较为锐利的吸收峰,但对于浸出渣相应位置的吸收峰变得平缓,峰值也变小。结合XRD分析结果,可以得出在浸出渣中SiO4四面体存在于MgNa2SiO4中、且浸出渣中硅酸盐含量较低是吸收峰变平缓和峰值减少的主要原因。在500 cm-1附近,Mg2SiO4红外光谱表现的是SiO4的弯曲振动,而浸出渣的红外光谱则呈现出Mg(OH)2晶格平行振动的特征。通过对浸出渣的红外光谱分析可以得出,Mg2SiO4与熔融碱反应后得到的浸出渣表现出明显的Mg—OH键和Mg(OH)2晶格的特征;并且SiO4的伸缩振动峰随着碱量的增加峰强逐渐减弱。可以推断Mg2SiO4经过碱熔融处理后,其中的Mg离子可以与NaOH中的Na离子交换,脱离其原有的硅酸盐阵列;且增加碱量有助于镁离子由Mg2SiO4转化成Mg(OH)2。
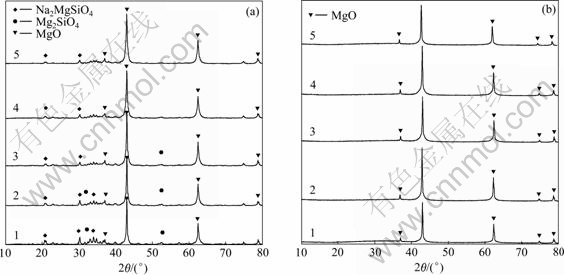
图3 在15 min、400 ℃条件下Mg2SiO4碱熔融水浸渣的XRD谱
Fig. 3 XRD patterns of water-washing residue of Mg2SiO4 after alkali fusion at 400 ℃ for 15 min with different ratios of NaOH to Mg2SiO4: (a) Samples for dropping down to molten NaOH; (b) Samples for mixing with solid NaOH; 1—10:1; 2—15:1; 3—20:1; 4—25:1; 5—30:1
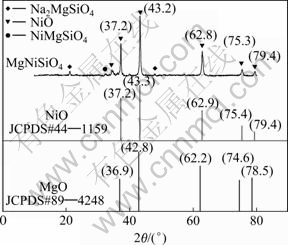
图4 在15 min、400 ℃条件下MgNiSiO4碱熔融后水浸渣的XRD谱
Fig. 4 XRD patterns of residue obtained from MgNiSiO4 after alkali fusion of NaOH at 400 ℃ for 15 min (data within brackets show values of 2θ corresponding to peaks in XRD patterns)
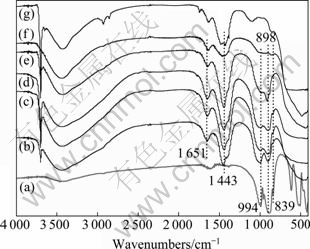
图5 在400 ℃、15 min条件下Mg2SiO4-NaOH体系碱熔融后水浸渣的红外光谱
Fig. 5 FT-IR spectra of residue of Mg2SiO4 after alkali fusion with NaOH with different mole ratios of NaOH to Mg2SiO4 at 400 ℃ for 15 min: (a) Mg2SiO4; (b) 10:1; (c) 15:1; (d) 20:1; (e) 25:1; (f) 30:1; (g) Mg(OH)2
图6所示为400 ℃、15 min 不同碱量分解MgNiSiO4的产物经过水浸后的水浸渣煅烧后的红外光谱。与Mg2SiO4碱熔融后得到水浸渣的结果类似,其红外光谱在3 424 cm-1附近的较宽吸收峰,对应其中吸附水中的O—H伸缩振动;但由于MgNiSiO4碱熔融后的水浸渣全部经500 ℃煅烧,所以在3 700 cm-1未出现与M—OH伸缩振动对应的锐利吸收峰。在 1 641 cm-1附近吸收峰应是镁镍橄榄石中的Mg—O的振动峰,而MgNiSiO4水浸渣的红外光谱在1 641 cm-1附近只有很弱的吸收峰;但在1 443 cm-1附近也出现明显的Mg—OH振动峰。MgNiSiO4在997和 884 cm-1可见SiO4的伸缩振动峰,与Mg2SiO4结果非常相近;水浸渣的红外光谱在该频率范围内出现平缓和较宽的吸收峰,可能是由于体系中同时存在的Na+,Ni2+和 Mg2+ 作用下使SiO4弯曲振动峰呈现合并现象。在500 cm-1附近,MgNiSiO4红外光谱表现的是SiO4的弯曲振动,而浸出渣的红外光谱在440 cm-1呈现Ni—OH弯曲振动[13],与煅烧后浸出渣的XRD中呈现的主要氧化物为NiO结果相对应。表明MgNiSiO4在400 ℃,不同碱量熔融反应15 min的过程中与NaOH优先交换的阳离子为Ni2+。
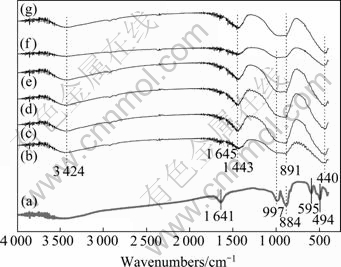
图6 MgNiSiO4-NaOH体系在400℃、15 min下碱熔融后水浸渣的红外光谱
Fig. 6 FT-IR spectra of residue of MgNiSiO4 after alkali fusion with NaOH with different mole ratios of NaOH to MgNiSiO4 at 400 ℃ for 15 min: (a) MgNiSiO4; (b) 10:1; (c) 15:1; (d) 20:1; (e) 25:1; (f) 30:1; (g) 40:1
2.4 碱熔融反应原位拉曼光谱分析
图7所示为NaOH和Mg2SiO4按照摩尔比4:1预先混料升至不同温度时体系的拉曼光谱图。其中: 25 ℃的拉曼光谱对应NaOH和Mg2SiO4混合体系在室温时的拉曼光谱以及室温时Mg(OH)2拉曼光谱,可以在1 074、3 564以及3 630 cm-1处看到NaOH特征振动峰;而795、824、859 cm-1处的振动峰归属于Mg2SiO4中的SiO4岛状特征振动峰[14-15]。当混合体系温度逐渐升高的过程中,NaOH在1 074 cm-1处的振动峰发生劈裂、以及劈裂峰合并、并且向低波数移动;由于随着NaOH与Mg2SiO4反应的进行,体系中NaOH逐渐消耗,使整个体系的拉曼光谱在1 074 cm-1附近的NaOH特征峰值也逐渐减少。熔融的NaOH对Mg2SiO4中SiO4岛状伸缩振动峰有明显的遮蔽效应,在795和824 cm-1振动峰强度减弱甚至消失;碱熔产物的SiO4岛状伸缩振动峰逐渐向低波数移动至803 cm-1附近,这与岛状SiO4键合的阳离子由钠离子过渡为镁离子相关,且镁的相对分子质量大于钠的相对分子质量。随着体系温度的升高和熔融碱的作用,岛状SiO4与阳离子的键合方式如图8所示(结合前面XRD结果)。
SiO4岛状伸缩振动峰经历Mg和Na离子共同作用下的振动峰宽化,且向低波数移动,最终产物Na4SiO4中SiO4岛状伸缩振动峰在803 cm-1[16]。同时发现,NaOH在1 074 cm-1处OH振动峰强度随着Na4SiO4中岛状SiO4于803 cm-1处伸缩振动峰的增强而减弱,这说明碱熔融过程NaOH逐渐消耗;虽然产物Mg(OH)2量会增加,但是Mg(OH)2在1 074 cm-1附近无拉曼活性特征峰。在体系温度升至400 ℃之后,高频区3 600 cm-1附近拉曼峰应主要对应Mg(OH)2中OH—的振动[17];但由于在反应过程中同时存在NaOH和Mg(OH)2,所以在高频区OH的拉曼特征峰出现明显宽化;Mg(OH)2在500 ℃以上将分解反应,生成MgO,所以在3 500 cm-1附近OH—的振动峰在 500 ℃以及更高的温度范围呈减弱趋势。
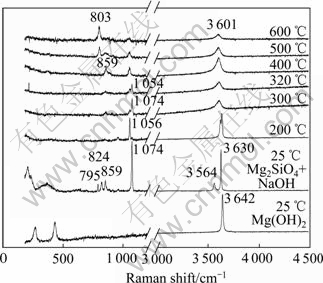
图7 NaOH和Mg2SiO4摩尔比为4:1时升至不同温度时的拉曼光谱
Fig. 7 Raman spectra of mixture of NaOH and Mg2SiO4 at different temperatures with mole ratio of NaOH to Mg2SiO4 of 4:1
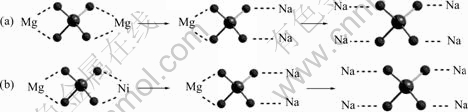
图8 硅酸镁碱熔融活化过程示意图
Fig. 8 Schematic illustration of alkali process of forsterite(a) and magnesium-nickel silicate(b)
3 结论
1) 利用水溶液沉淀结合固相反应法依次合成Mg2SiO4和MgNiSiO4。XRD的分析结果表明:合成Mg2SiO4和MgNiSiO4具有类似的晶体结构,镍离子以异质同相的方式存在于Mg2SiO4橄榄石相中。
2) 对Mg2SiO4碱熔融后的水浸渣XRD和红外光谱FT-IR分析表明,NaOH对Mg2SiO4中Mg2+的替换逐次进行,其中间产物为Na2MgSiO4。对碱熔融过程的拉曼光谱在线分析显示,碱熔融过程中与岛状SiO4结合的阳离子Mg2+逐次由Na+替换,对应岛状SiO4伸缩振动模式拉曼峰向低波数移动;Mg离子经过碱熔融过程可以脱离SiO4阵列,以Mg(OH)2 或MgO的形式从其硅酸盐中得以释放。
3) 对MgNiSiO4碱熔融后的水浸渣XRD和红外光谱FT-IR分析结果表明,碱熔融过程中Na+对MgNiSiO4中阳离子的替换是有择优取向的,将首先替换其中的Ni2+,生成中间产物Na2MgSiO4。
REFERENCES
[1] 刘 岩, 翟玉春, 王 虹. 镍生产工艺研究进展[J]. 材料导报, 2006, 20(3): 79-81, 96.
LIU Yan, ZHAI Yu-chun, WANG Hong. Research on production process of nickel[J]. Materials Review, 2006, 20(3): 79-81, 96.
[2] 彭容秋, 任鸿九, 张训鹏. 镍冶金[M]. 长沙: 中南大学出版社, 2005: 1-5.
PENG Rong-qiu, REN Hong-jiu, ZHANG Xun-peng. Nickel metallurgy[M]. Changsha: Central South University Press, 2005: 1-5.
[3] 曹异生. 国内外镍工业现状及前景展望[J]. 世界有色金属, 2005(10): 67-71.
CAO Yi-sheng. Status quo and prospects of nickel industry at home and abroad[J]. World Nonferrous Metals, 2005(10): 67-71.
[4] 何焕华. 世界镍工业现状及发展趋势[J]. 有色冶炼, 2001, 12(6): l-3.
HE Huan-hua. Present situation and development trend of world nickel industry[J]. China Nonferrous Metallurgy, 2001, 12(6): 1-3.
[5] 刘 瑶, 丛自范, 王德全. 对低品位镍红土矿常压浸出的初步探讨[J]. 有色矿冶, 2007, 23(5): 28-30.
LIU Yao, CONG Zi-fan, WANG De-quan. Primary probe into normal atmospheric leaching of low-nickel laterites[J]. Non-ferrous Mining and Metallurgy, 2007, 23(5): 28-30.
[6] 曹来宗, 刘代俊, 高丽花, 刘长虹, 沈 茜. 亚熔盐法浸取钒的实验研究[J]. 钢铁钒钛, 2008, 29(2): 1-4.
CAO Lai-zong, LIU Dai-jun, GAO Li-hua, LIU Chang-hong, SHEN Qian. Experimental study on leaching vanadium by sub-molten salt method[J]. Iron Steel Vanadium Titanium, 2008, 29(2): 1-4.
[7] ABDELKADER M, DAHER A, EL-KASHEF E. Novel decomposition method for zircon[J]. Journal of Alloys and Compounds, 2008, 460(1/2): 577-580.
[8] HIDETSUGU M. Extraction of silicon dioxide from waste colored glasses by alkali fusion using sodium hydroxide[J]. Journal of the Ceramic Society of Japan, 2003, 111(1294): 376-381.
[9] TSAI M T. Synthesis of nanocrystalline enstatite fiber via alkoxide sol-gel process[J]. Journal of the American Ceramic Society, 2005, 88(7): 1770-1772.
[10] WU Xiang-feng, HU Guo-sheng, WANG Biao-bing, YANG Yun-feng. Synthesis and characterization of superfine magnesium hydroxide with monodispersity[J]. Journal of Crystal Growth, 2008, 310: 457-461.
[11] 尤静林, 蒋国昌, 徐匡迪. 二硅酸钠晶体、玻璃及其熔体结构的拉曼光谱研究[J]. 光谱学与光谱分析, 2000, 20(6): 797-799.
YOU Jing-lin, JIANG Guo-chang, XU Kuang-di. High temperature Raman spectroscopic study of the structure of sodium disilicate crystal, glass and its melt[J]. Spectroscopy and Spectral Analysis, 2000, 20(6): 797-799.
[12] ?RODA M, PALUSZKIEWICZ C. The structural role of alkaline earth ions in oxyfluoride aluminosilicate glasses-infrared spectroscopy[J]. Vibrational Spectroscopy, 2008, 48(2): 246-250.
[13] 何则强, 孙新阳, 熊利芝, 刘文萍, 陈 上, 吴显明, 黄可龙. 均匀沉淀法制备NiO超细粉末及其电化学性能[J]. 中国有色金属学报, 2008, 18(z1): 301-305.
HE Ze-qiang, SUN Xin-yang, XIONG Li-zhi, LIU Wen-ping, CHEN Shang, WU Xian-ming, HUANG Ke-long. Preparation and electrochemical properties of superfine NiO powders by homogeneous precipitation method[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(z1): 301-305.
[14] 蒋国昌, 尤静林, 吴永全, 侯怀宇, 陈 辉. 硅酸盐熔体微结构单元的探讨[J]. 地质地球化学, 2003, 31(4): 80-86.
JIANG Guo-chang, YOU Jing-lin, WU Yong-quan, HOU Huai-yu, CHEN Hui. A discussion on the micro-structural units of silicate melt[J]. Geology-geochemistry, 2003, 31(4): 80-86.
[15] KOLESOV B A, TANSKAYA J V. Raman spectra and cation distribution in the lattice of olivines[J]. Materials Research Bulletin, 1996, 31(8): 1035-1044.
[16] LIN Chung-cherng, CHEN Shin-fan, LIU Lin-gun. Anionic structure and elasticity of Na2O-MgO-SiO2 glasses[J]. Journal of Non-Crystalline Solids, 2007, 353(4): 413-425.
[17] WALRAFEN G E, DOUGLAS R T W. Raman spectra from very concentrated aqueous NaOH and from wet and dry, solid, and anhydrous molten, LiOH, NaOH, and KOH[J]. J Chem Phys, 2006, 124: 114504.
(编辑 龙怀中)
基金项目:国家重点基础研究发展计划资助项目(2007CB613603);上海市现代冶金与材料制备重点实验室开放课题资助项目(SELF-2009-02)
收稿日期:2011-01-07;修订日期:2011-06-07
通信作者:许 茜,教授,博士;电话:024-83684943;E-mail: qianxu201@mail.neu.edu.cn