Statistical evaluation of flotation and entrainment behavior of an artificial ore
来源期刊:中国有色金属学报(英文版)2012年第1期
论文作者:Taki GULER Unal AKDEMIR
文章页码:199 - 205
关键词:天青石;浮选时间;捕收剂浓度;起泡剂类型;夹带;粒径;方差分析
Key words:celestite; flotation time; collector concentration; frother type; entrainment; particle size; two-way ANOVA
摘 要:研究浮选参数对泡沫浮选脉石回收率的影响,测试了pH=10时起泡剂类型和捕收剂用量对浮选性能的影响。采用方差分析了测试结果。在用油酸钠作捕收剂的情况下,亲水性矿物的浮选主要是由于Ca+2离子对石英的活化产生的疏水性而导致的夹带作用所引起的;夹带和矿泥包裹层对浮选也有一定的影响。在浮选产物中脉石的夹带程度随着矿石颗粒粒度的减小而增加。实验结果表明,通过增大捕收剂用量、缩短浮选时间,可以减小水流夹带作用,提高浮选的选择性。
Abstract:
The role of several parameters on gangue recovery in froth was investigated. Effects of frother type and collector dosage on flotation performance were tested at pH 10. The results were evaluated statistically by two-way analysis of variance without replicates, sample range and sample standard deviation. Hydrophilic mineral was recovered mainly by entrainment through hydrophobization of Ca+2-activated quartz from tap-water in the presence of Na-oleate as collector; entrapment and slime coating were also proposed as recovery mechanisms in minority. Degree of gangue-entrainment in froth product increases by reduction in liberation size. The experimental results state that selectivity would be improved by increasing collector concentration and reducing the flotation time.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 199-205
Taki G?LER1, ?nal AKDEM?R2
1. Mining Engineering Department, Mu?la University, 48170 Mu?la, Turkey;
2. Mining Engineering Department, Cumhuriyet University, 58140 Sivas, Turkey
Received 14 March 2011; accepted 26 May 2011
Abstract: The role of several parameters on gangue recovery in froth was investigated. Effects of frother type and collector dosage on flotation performance were tested at pH 10. The results were evaluated statistically by two-way analysis of variance without replicates, sample range and sample standard deviation. Hydrophilic mineral was recovered mainly by entrainment through hydrophobization of Ca+2-activated quartz from tap-water in the presence of Na-oleate as collector; entrapment and slime coating were also proposed as recovery mechanisms in minority. Degree of gangue-entrainment in froth product increases by reduction in liberation size. The experimental results state that selectivity would be improved by increasing collector concentration and reducing the flotation time.
Key words: celestite; flotation time; collector concentration; frother type; entrainment; particle size; two-way ANOVA
1 Introduction
Froth flotation is a separation technique applied to various types of ores using differences in the interfacial properties of mineral surfaces constituting ore. Although the degree of hydrophobicity is the major criterion on flotation performance, separation efficiency is also related with particle size. So, liberation size should be taken into consideration to reduce fine gangue recovery in froth.
Entrainment is the primary mechanism in gangue recovery in froth product. Finely sized liberated particles are carried out in thin water layer surrounding air bubble, and recovered in concentrate launder regardless of the degree of hydrophobicity of particles [1-3]. The mass of particles decreases at lower liberation size, and hence the effect of gravity becomes negligible, resulting in tenddency to behave more and more as part of the liquid phase. Therefore, degree of entrainment is directly related with water recovery, and decreases at coarser sizes. On the other hand, coarse grinding (lower degree of liberation) means that unliberated locked particles are attached to air bubbles by their hydrophobic parts, and transferred to concentrate launder, causing lower separation efficiency [2, 4-6].
Naturally hydrophobic minerals can be floated without the addition of surfactant. However, in most applications, collector is required to render valuable part of ore hydrophobic. On the other hand, frother is used to obtain stable froth to carry the hydrophobic mineral particles into the concentrate launder. Type and amount of collector and frother are strongly related with entrainment of gangue in froth and recovery of valuable minerals. SRIPRIYA et al [7] stated that the degree of entrainment and flotation rate of coal is increased by increasing collector concentration at higher frother dosage. However, they also proposed that the net recovery of solid decreased with increasing the collector consumption in the presence of lower frother dosage due to froth overloading. G?LER et al [8] studied the role of type and amount of frother on the flotation of celestite-calcite ore, and observed an increase in celestite recovery at higher frother dosages. Concentrate grade did almost not change up to a certain frother concentration, whereas it decreased sharply at higher dosages due to entrainment. The work of SUBRAHMANYAM and FORSSBERG [9] indicated that a better grade was obtained in the increasing order pine oil, polypropylene glycol, triethoxybuthane and MIBC in copper ore flotation and these frothers influenced gangue and water recovery considerably.
The two-way analysis of variance (two-way ANOVA) is an important statistical test in the evaluation of experimental results. It is applied to studying the effects of independent variables on dependent factor by comparing the means of populations to determine whether variation in the independent variable affects the means of dependent factor. Experimental results may also be evaluated by descriptive statistical data such as sample range and sample standard deviation. Increase in the value of these data indicates the increase in separation of experimental results from each other. These data direct at basic statistical results [10].
This work was performed to understand the role of particle size, collector and frother on the entrainment of fine gangue and recovery of valuable minerals. Celestite (SrSO4), sulfate of strontium, was employed as hydrophobic mineral. It is the chief commercial source of the element strontium and its compounds. It is traditionally concentrated using Na-salt of oleic acid. Quartz (SiO2) was studied as hydrophilic gangue mineral, which is generally encountered in most of the flotation applications as gangue.
2 Experimental
2.1 Materials
High purity quartz (99.2% SiO2) supplied from Cami? Mining Co. (Kuruca?ile, Turkey), and a celestite concentrate having 96.3% SrSO4 grade from Barit Maden Co. (Sivas, Turkey), were used throughout the experimental works. Quartz samples with different sizes (d<38 μm and 38-63 μm), and celestite samples (38-63 μm) were prepared by screening after crushing and grinding. The tests were carried out using 100 g solid in a laboratory type flotation cell of 1.2 L capacity.
Na-oleate was employed as collector when it was necessary. The frothers tested were pine-oil, MIBC, Flotanol F (alkyl polypropylene glycol ether, a commercial product of Clariant, Co.) and Aerofroth-65 (polypropylene glycol, a commercial product of Cytec, Co.). An impeller speed of 1 100 r/min was applied to all flotation tests. Conditioning time for Na-oleate and frothers are 3 min and 1 min, respectively.
2.2 Flotation tests
Flotation experiments were conducted on a Denver laboratory type flotation machine. Tap water was used in experimental work. Chemical analysis of tap water was given elsewhere [11]. Pulp pH was adjusted to 10 by NaOH. Flotation tests of single quartz (100 g), single celestite (100 g) and, artificial ore of quartz (<38 μm) and celestite (38-63 μm) mixtures one to one by mass (50 g celestite + 50 g quartz) were performed under certain experimental conditions. The SrSO4 grade of froth product of artificial ore was determined by sieving this material (>38 μm) on a sieve with sieve size of 38 μm. The mass of materials to the total mass of froth product gives the grade of celestite in froth. The recoveries of both materials were also calculated from the same mass.
2.3 Entrainment factor
The entrainment factor for hydrophilic quartz was calculated using Kirjavainen’s equation as [2]:
![]() (1)
(1)
where Ri and Rw are quartz and water recoveries, respectively, and Pi is entrainment factor.
2.4 Statistical analysis
The experimental results were analyzed using two-way analysis of variance (two-way ANOVA) test without replicates. Two-way ANOVA test was used to investigate the effects of two independent variables (column factor and row factor) on one dependent factor by comparing the means of populations. Columns and rows represent various values or levels of independent factors, respectively. This test was applied to elucidating whether variation in the column (or row) variable affected the column (or row) means. To test these hypotheses, the experimental results given in Table 1 were used to construct Table 2 [10].
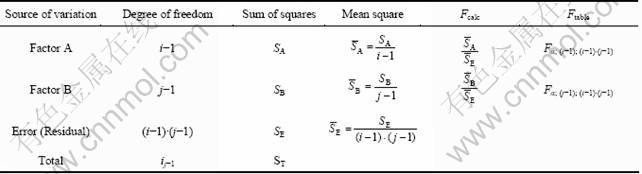
The sum of squares of factor A (SA), factor B (SB), error (SE) and total (ST) were calculated by using equations (2)-(5). The calculated F values (Fcalc) were compared with the critical F values (Ftable) obtained from F-tables for (1-α) confidence (or for significance level of α): If the calculated value is greater than the critical one, then the independent variable has significant influence on the dependent variable at a level of 100×(1-α)% confidence. Two-way ANOVA tests were performed using MS Excel 2003 software program. The probability (p) values of independent variables were also obtained using this software. If the calculated “p” value is lower than the significance level α, then the influence of independent variable on the dependent variable is statistically significant, and vice-versa.
Table 1 Data arrangement for two-factor experiment
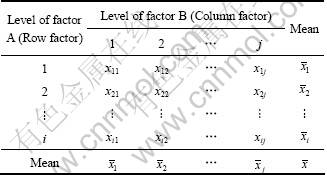
Table 2 ANOVA table for two independent factors
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
ST=SA+SB+SE (5)
The experimental data were also evaluated by computing the sample range, SR (Eq. (6)) and sample standard deviation, s (Eq. (7)).
![]() (6)
(6)
![]() (7)
(7)
where xmin and xmax are the minimum and maximum experimental results in the group, respectively; s is the sample standard deviation; xi is the ith variable of the sample; n is the number of data in the group; ![]() is the arithmetic mean.
is the arithmetic mean.
3 Results and discussions
The results of flotation experiments carried out with quartz (38 μm), quartz (38-63 μm) and celestite (38-63 μm) at various Na-oleate concentrations in a single mineral flotation system are given in Table 3 and Fig. 1. The collector improved the celestite recovery, resulting in a significant sample range, SR (51.170) and sample standard deviation, s (23.977). The increase in recovery became sharper up to 500 g/t Na-oleate, and then a gradual increase was observed. So, the governing mechanism for celestite recovery is obviously the hydrophobicity. On the other hand, a certain amount of celestite was also recovered in the absence of collector, which would be attributed to entrainment [1, 6].
Quartz demonstrates different flotation behavior. Na-oleate did almost not affect quartz recovery in the flotation of sample (38-63 μm), causing considerably smaller statistical results (SR=4.870; s=2.084). Only about 10% quartz, as an average, was recovered in the froth phase. On the other hand, a slight improvement occurred in the processing of finely sized fraction, which resulted in a certain increase in “SR” and “s” values. Quartz recovery reached approximately 22% in the flotation with 500 g/t Na-oleate. These results would be explained with entrainment: entrainment of hydrophilic particles increases with decreasing size [3]. An enhanced recovery of finely-sized hydrophilic particles in the froth phase would be related with the frothing ability of Na-oleate in addition to collecting action, and therefore, with the formation of more stable froth entraining the fine hydrophilic particles in aqueous phase present between bubbles [12].
Table 3 Flotation of celestite and quartz at various Na-oleate concentrations in single mineral system, and statistical evaluation
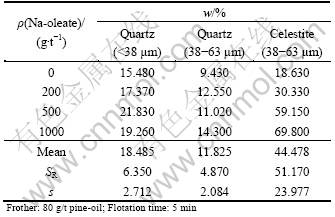
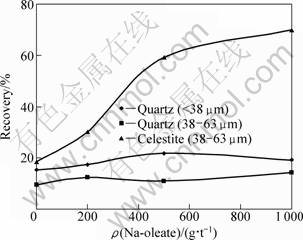
Fig. 1 Flotation of celestite and quartz at various Na-oleate concentrations in single mineral system (Frother pine-oil: 80 g/t; Flotation time: 5 min)
The experimental works were conducted using hard tap water (ρ(Ca+2)≈200 mg/L≈5×10-3 mol/L; ρ(Na+)≈ 30 mg/L≈1.33×10-3 mol/L) in the laboratories of Cumhuriyet University [11]. Therefore, entrainment might not be the sole mechanism. Especially, fine quartz particles might also be accidentally activated in minority with dissolved Ca+2-ions in the tap water [13, 14]. TRAHAR [15] proposed that only a low hydrophobicity was necessary for fine particles to be captured by the true flotation process as compared with coarser ones. COOKE and DIGRE [13] proposed that the concentration of calcium ion should be greater than ρ(Na+)×10-4 mol/L for Ca+2-activation of quartz. Indeed, increase in the recovery up to 500 g/t oleate addition might result from the adsorption of Ca+2-ions on quartz particles, which promotes the interaction of activated-quartz with oleate ions inducing the formation of hydrophobic surface [16]. Calcium ion adsorption on quartz decreases to some extent in the presence of relatively high oleate concentration possibly due to Ca+2-oleate interaction, which would affect the simultaneous Ca+2-quartz attraction [14].
Entrainment occurs without a direct attachment of particle to the bubble. The mechanism of particle entrainment in the launder is related with the water recovery and flotation time. Therefore, the relationship between gangue and water recovery was also investigated as a function of flotation time (Table 4, Fig. 2). Nearly linear relationship between water and quartz recoveries is a well-known indicator of entrainment for quartz [5, 17-19]. As stated in definition of the term “entrainment”, increase in the water recovery promotes gangue entrainment in froth product. On the other hand, statistical values given in Table 4 indicate that increase in recoveries of quartz and water with respect to flotation time is not so proportional; change in SR and s values with flotation time states that there is a strong correlation between quartz and water recoveries up to 3 min froth scrapping. Similarly, entrainment factor increased almost linearly. However, with longer flotation time (5 min), SR (17.430) and s (12.325) became significantly larger, and Pi value decreased from 0.629 for 3 min to 0.511 for 5 min. Finer particles tend to behave as part of liquid phase with decreasing the size. So, the particles with smaller sizes, and hence lower particle masses, will be recovered initially in water constituting the froth phase with flotation gas. In conclusion, the capturing possibility of left heavy-large hydrophilic gangue particles in froth phase reduces, and hydrophilic gangue recovery rate decreases with the time [3, 19]. Entrainment factor for quartz (<38 μm) was calculated as 0.511 for 5 min flotation, whereas it was only 0.340 for particles of 38-63 μm.
Table 4 Recoveries of quartz (<38 μm) and water depending on flotation time in single mineral system, and statistical evaluation
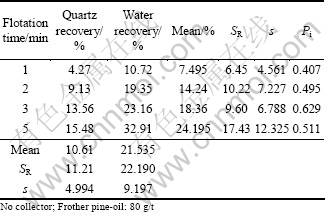
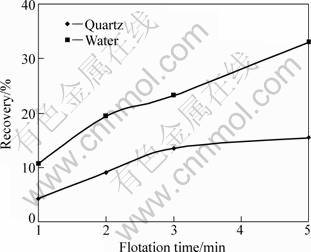
Fig. 2 Recoveries of quartz (<38 μm) and water depending on flotation time in single mineral system (No collector; Frother pine-oil: 80 g/t)
ANOVA table was also prepared for quartz and water recoveries as a function of flotation time (Table 5). Two-way ANOVA test proposed that the recovery responses of components (quartz and water) were statistically different from each other: Fcalc=22.174>Ftable=10.128 and p=0.0118<α=0.05. Similar result was also proposed above according to SR (SRWater=22.190>SRQuartz=11.210) and s (sWater=9.197> sQuartz=4.994) values (Table 4). However, p value (0.051) for the flotation time factor is negligibly higher than significance level (α=0.05), while the calculated F value (9.174) is slightly lower than the critical one (9.277). Such values resulted possibly from the difference of recovery values with longer flotation time. Therefore, flotation time would also be suggested to have important influence on recovery.
The flotation and entrainment data for fine quartz (<38 μm) with four types of frothers are given in Table 6. A survey of the data in the table indicated that frother type was effective to some extent on both water and quartz recoveries and entrainment factors. The lowest quartz recovery (13.270% SiO2) and entrainment factor (0.416) were obtained with Flotanol-F, while these values became as 19.930% SiO2 and 0.588 with MIBC, respectively. In other words, better gangue recovery and entrainment factor values were obtained by Flotanol F, while Aerofroth-65 gave a minimum water recovery with considerably bad entrainment factor (0.556). AKDEMIR et al [5] investigated the role of frothers on gangue entrainment in talc-calcite separation, and obtained the minimum entrainment with Flotanol F. They pointed out that the optimum frother type would obviously differ for every particular system. ANOVA test was also made to clarify statistically the effect of frother type on the recoveries of components given in Table 6. However, statistically significant difference would not be obtained at a level of 95% confidence possibly due to the considerably closer SR and s values to each other. Therefore, ANOVA table is not presented here.
Table 5 ANOVA table for effect of flotation time on recoveries of quartz (<38 μm) and water recovery in single mineral system (α=0.05)
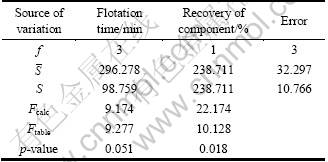
Table 6 Effect of frother type on recoveries of quartz (<38 μm) and water in single mineral system, and statistical evaluation

Further studies were carried out with an artificial ore at various Na-oleate concentrations (Table 7, Fig. 3). Ore was prepared from mixing quartz (<38 μm, gangue mineral) and celestite (38-63 μm) (valuable mineral) one to one by mass. Maximum SR and s values were calculated for the recovery of hydrophobic mineral; meanwhile these values were lower for quartz and water. Since the main mechanism for celestite flotation is hydrophobicity imparted by collector addition. Oleate addition increased celestite recovery, much beyond quartz recovery, resulting in the higher grade of concentrate. A sharp increase in recovery up to 500 g/t Na-oleate, and then a gradual increase was observed. However, collectorless flotation of valuable mineral at a recovery level of 18.280% should be considered the presence of different recovery mechanisms, such as entrainment, other than hydrophobization [17, 18]. The use of collector at over-dosage (1000 g/t) did almost not cause any significant results in the recovery of components and celestite grade.
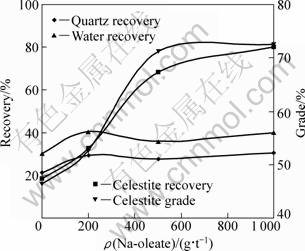
Fig. 3 Flotation results of an artificial ore of celestite (38-63 μm) and quartz (<38 μm) (1:1) at various Na-oleate concentrations (Frother pine-oil: 80 g/t; Flotation time: 5 min)
Table 7 Flotation results of an artificial ore of celestite (38-63 μm) and quartz (<38 μm) (1:1) at various Na-oleate concentrations, and statistical evaluation (Frother: 80 g/t pine-oil; Flotation time: 5 min)
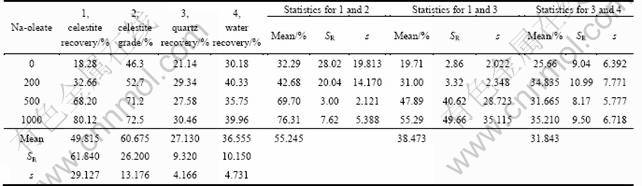
Data in Table 7 signify that the effect of collector concentration on the recovery of quartz and water is restricted to a few percentages, and might not be taken into consideration in the processing of artificial ore. On the other hand, one can observe from the comparison of Fig. 1 and Fig. 3 that quartz recovery of artificial ore of quartz-celestite mixture is higher than that of single quartz system. Recovery of 15%-20% for single mineral system rises to 20%-30% for mixture system. So, it can be concluded from the experimental results that quartz would mainly be recovered by entrainment even though hydrophobization of Ca-activated quartz by oleate, entrapment and slime coating might also be effective on recovery in minority [13, 14, 17, 19, 20].
Final experimental works were conducted with artificial celestite-quartz ore mixing one to one by mass to investigate the combined effects of flotation time and collector concentration on the recovery and selectivity of separation. The results presented in Table 8 show that more selective products could be obtained at 1000 g/t level by reducing flotation time. While a celestite concentrate of 71.21% SrSO4 was obtained by combination of 500 g/t collector dosage and 5 min flotation time; a high-grade (76.65% SrSO4) product was obtained at nearly 70% celestite recovery level by reducing flotation time and increasing collector concentration. This is probably due to the increased difference between the flotation rates of hydrophobic celestite and hydrophilic quartz [17]. Increasing the flotation rate of hydrophobic mineral by raising collector concentration at a shorter flotation time may be a useful way of increasing selectivity of separation. This finding is consistent with the work of AKDEMIR et al [5].
Table 8 Recoveries and grades of froth product of artificial ore of celestite (38-63 μm) and quartz (<38 μm) one to one by mass at various conditions (Frother pine-oil: 80 g/t)

4 Conclusions
1) The primary governing recovery mechanisms for valuable-hydrophobic and gangue-hydrophilic minerals were hydrophobicity and entrainment, respectively. Degree of entrainment increases at finer liberation sizes. It is also influenced by the type of frother.
2) Flotation time affects gangue entrainment and water recovery: gangue recovery rate decreases with the time due to skimming-off the finer particles first. As a whole, a shorter froth skimming period by increasing the collector concentration would be effective to increase selectivity of separation due to the difference between the flotation rates of minerals constituting ore.
3) In the processing of an ore, entrainment would not be the sole mechanism in the recovery of hydrophilic gangue in froth. Other mechanisms, like accidental activation, slime coating and entrapment, would also be effective. Accidental activation due to chemical composition of process water might adversely affect selectivity.
References
[1] G?LSOY ? Y. A simple model for the calculation of entrainment in flotation [J]. Korean Journal of Chemical Engineering, 2005, 22(4): 628-634.
[2] KIRJAVAINEN V M. Application of a probability model for the entrainment of hydrophilic particles in froth flotation [J]. International Journal of Mineral Processing, 1989, 27: 63-74.
[3] SZYSZKA D, GLAPIAK E, DRZYMALA J. Entrainment-flotation activity of quartz in the presence of selected frothers [J]. Physicochemical Problems of Mineral Processing, 2008, 42: 85-90.
[4] AKDEMIR ?, G?LER T. Role of some physical variables on gangue and water recovery in froth[C]//?ZBAYO?LU G, HO?TEN C, ATALAY M ?, HI?YILMAZ C, AROL A ?. Mineral Processing on the verge of the 21st Century: Proceedings of the 8th International Mineral Processing Symposium in Antalya. Rotterdam: A. A. Balkema, 2000: 257-261.
[5] AKDEMIR ?, G?LER T, YLDIZTEK?N G. Flotation and entrainment behavior of minerals in talc-calcite separation [J]. Scandinavian Journal of Metallurgy, 2005, 34: 241-244.
[6] CILEK E C. The effect of hydrodynamic conditions on true flotation and entrainment in flotation of a complex sulphide ore [J]. International Journal of Mineral Processing, 2009, 90: 35-44.
[7] SRIPRIYA R, RAO P V T, ROY CHOUDHURY B. Optimisation of operating variables of fine coal flotation using a combination of modified flotation parameters and statistical techniques [J]. International Journal of Mineral Processing, 2003, 68: 109-127.
[8] G?LER T, H??YILMAZ C, AKDEM?R ?. Effect of frothers in flotation of celestite-calcite ore from Sivas-Nas?r[C]//ATALAY?, H??YILMAZ C, ERSAYIN S, TERCAN E. 16th Mining Congress of Turkey. Ankara: The Chamber of Mining Engineers of Turkey, 1999: 391-393.
[9] SUBRAHMANYAM T V, FORSSBERG K S E. Froth characteristics and grade-recovery relationship in the flotation of lead-zinc and copper ores [J]. Minerals Engineering, 1988, 1: 41-52.
[10] MONTGOMERY D C, RUNGER G C. Applied statistics and probability for engineers [M]. John Wiley and Sons, Inc., 2007: 768.
[11] Governorship of Sivas. Environmental Status Report of Sivas Province—2006 (in Turkish) [EB/OL]. http://www2.cedgm. gov.tr/icd_raporlari/sivasicd2006.pdf.
[12] BULATOVIC S M. Handbook of flotation reagents: Volume 1 [M]. Netherlands: Elsevier Publishers, 2007: 446.
[13] COOKE S R B, DIGRE M. Studies on activation of quartz with calcium ion [J]. Transactions AIME, 1949, 184: 299-305.
[14] MALATI M A, ESTEFAN S F. Activation of quartz by alkaline earth cations in oleate flotation [J]. Journal of Applied Chemistry, 1967, 17: 209-212.
[15] TRAHAR W J. A rational interpretation of the role of particle size in flotation [J].International Journal of Mineral Processing, 1981, 8: 289-327.
[16] VENKATACHALAM S, MALLIKARJUNAN R. Activation and deactivation studies with copper(II) ions on the soap flotation of quartz [J]. Journal of Applied Chemistry, 1965, 15: 605-610.
[17] GEORGE P, NGUYEN A V, JAMESON G J. Assessment of true flotation and entrainment in the flotation of submicron particles by fine bubbles [J]. Minerals Engineering, 2004, 17: 847-853.
[18] YIANATOS J, CONTRERAS F, DIAZ F, VILLANUEVA A. Direct measurement of entrainment in large flotation cells [J]. Powder Technology, 2009, 189: 42-47.
[19] ZHENG X, JOHNSON N W, FRANZIDIS J P. Modeling of entrainment in industrial flotation cells: Water recovery and degree of entrainment [J]. Minerals Engineering, 2006, 19: 1191-1203.
[20] GONG J, PENG Y, BOUAJILA A, OURRIBAN M, YEUNG A, LIU Q. Reducing quartz gangue entrainment in sulphide ore flotation by high molecular weight polyethylene oxide [J]. International Journal of Mineral Processing, 2010, 97: 44-51.
Taki G?LER1, ?nal AKDEM?R2
1. Mining Engineering Department, Mu?la University, 48170 Mu?la, Turkey;
2. Mining Engineering Department, Cumhuriyet University, 58140 Sivas, Turkey
摘 要:研究浮选参数对泡沫浮选脉石回收率的影响,测试了pH=10时起泡剂类型和捕收剂用量对浮选性能的影响。采用方差分析了测试结果。在用油酸钠作捕收剂的情况下,亲水性矿物的浮选主要是由于Ca+2离子对石英的活化产生的疏水性而导致的夹带作用所引起的;夹带和矿泥包裹层对浮选也有一定的影响。在浮选产物中脉石的夹带程度随着矿石颗粒粒度的减小而增加。实验结果表明,通过增大捕收剂用量、缩短浮选时间,可以减小水流夹带作用,提高浮选的选择性。
关键词:天青石;浮选时间;捕收剂浓度;起泡剂类型;夹带;粒径;方差分析
(Edited by YANG Hua)
Corresponding author: Taki G?LER; Tel: +90-252-2111654; Fax: +90-252-2111912; E-mail: takiguler@mu.edu.tr
DOI: 10.1016/S1003-6326(11)61161-8

