
Wear behavior of Al-Si alloy matrix composites reinforced by
γ-Al2O3 decomposed from AACH
FU Gao-feng(付高峰)1, ZHANG Jing-xin(张景新)2, LIU Ji(刘 吉)1, WANG Zhao-fei(王肇飞)1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. Fushun Aluminum Co Ltd, Fushun 113001, China
Received 28 February 2006; accepted 21 September 2006
Abstract: The Al-Si alloy matrix composite reinforced by γ-Al2O3 particles was produced by adding NH4AlO(OH)HCO3(AACH) into molten Al-Si alloy at 850 ℃. During stirring γ-Al2O3 particles are formed by the decomposing reaction of AACH. It is found that the γ-Al2O3 particles distribute more uniformly in the matrix by adding AACH than by adding γ-Al2O3 directly. The wear tests show that the volume loss of the unreinforced Al-Si alloy matrix is about 3 times larger than that of the γ-Al2O3 reinforced composites and that of the composites fabricated by adding γ-Al2O3 is larger than that by adding AACH.
Key words: Al-Si alloy matrix composite; Al2O3 particles; microstructure; wear
1 Introduction
The particles reinforced aluminium alloy matrix composites, which possess high-specific elastic modulus and strength, are being widely used in the aerospace and automobile industries[1-4]. Traditionally, the Al alloy matrix composites are produced by directly adding reinforcements into the matrix. In general, the uniform distribution of reinforcement particles inside the matrix, good wettability between the particle and matrix, and suitable bonding strength of the reinforcement/matrix interface are thought to be the major factors controlling the properties of this kind of materials[5-7]. To over- come these problems, in-situ technique referred to as reaction process has been proposed to fabricate composites by in situ formation of the A12O3 particles as reinforcements within the matrix via chemical reactions [8].
One commonly adopted in situ method involves the reaction between metal oxides, such as CuO, ZnO, SiO2, Fe2O3, MnO2, and TiO2[9-13], and Al to produce Al2O3 particles or whisker reinforcements. The reaction in the process can be represented as follows: MO+Al→Al2O3+M, where Al2O3 is a desired reinforcement, but M is also produced in the process. M can enter the Al matrix as alloying elements, hence the alloying strengthening is obvious. It is worthy to notice that the amount of each alloy element in Al alloy has a limit. When higher level of Al2O3 is desired in the process, larger amount of M is inevitably presented, thus it is conceivable that the deterioration of materials properties can not be avoided due to the larger amount of M in the matrix than in ordinary Al alloy. In most of the published studies on in-situ Al2O3/Al composites, special attention has been given to the effect of Al2O3, but less attention to the effect of M on the properties of composites. In order to optimize the properties, the existence of M must be taken into consideration, i.e. besides designing the ceramic reinforcement, the matrix must also be designed.
In order to gain higher level of Al2O3, one compound can be introduced into the reaction without M formation. In this paper, the technology of manufacturing Al-Si alloy matrix composites reinforced by γ-Al2O3 particles decomposed from NH4AlO(OH)HCO3 added to the molten Al-Si alloy was described, the microstructure was evaluated by scanning electron microscopy(SEM) and the wear behavior was studied.
2 Experimental
In order to examine the fundamentals of the in situ process with respect to supplied AACH powder, differential thermal analysis(DTA) on the AACH powder was firstly carried out at a heating rate of 10 ℃/min in argon atmosphere. In the next experiment, the powders of reinforcement were immersed into molten Al-Si alloy at 850 ℃ to fabricate the Al2O3/Al-Si composit.
Al-Si alloy with the composition of 2.6%Cu, 10.4%Si and rest Al (mass fraction, %) was supplied as the matrix metal. The raw material of reinforcement was NH4AlO(OH)HCO3 (AACH) (99.5% purity) and super- fine γ-Al2O3 (<100 nm, 99.7% purity) was used as comparing reinforcement. The amount of the powder of AACH used was 8% of the amount of Al2O3 (mass fraction). After melting, the molten metal was heated continuously and stirred at a rotating speed of 400 r/min at 850 ℃. The preheated powder of AACH was gradually added to the molten metal surface. As a result, an in situ formation of Al2O3 from AACH occurred within the metal matrix, and a molten composite formed. As the reactions were completed, the agitating was kept for a while to let the gas out and the molten was then cast in the form of cylinders with 30 mm in diameter and 50 mm in length. From these cylinders, wear test pieces were machined. Parallel studies of composites prepared by adding superfine γ-Al2O3 were performed under the same testing conditions.
Specimens for SEM observations were taken from the as-cast materials. The specimens were polished in a conventional manner and observed on SSX-550 scanning electron microscope(SEM).
Wear tests were carried out using a computer- controlled pin-on-disk tester with the aluminum alloy composites as the pin and GCr15 steel as the disk. The disk specimen was 70 mm in diameter and 5 mm in thickness. The pin test piece had a flat surface with a diameter of 6 mm. The diameter of the wear track was 60 mm. The surfaces of both the disk and the pin were polished with 1500# emery paper. The mass loss was measured after ultrasonic cleaning of the specimen in acetone, using a precision balance having a sensitivity of 0.01 mg. The measured mass loss was converted into volume loss by their corresponding density.
3 Results and discussion
3.1 Microstructure
The typical microstructures of the matrix and the composites are presented in Fig.1. Figs.1(b) and (c) show the microstructures of the composites prepared by adding AACH powder and the traditional composites by adding superfine γ-Al2O3 particles respectively.
The unreinforced Al-Si alloy contains many flaky and coarse acicular eutectic Si in the matrix(Fig.1(a)). In the composites, the amount of flaky eutectic Si is lower and the acicular eutectic Si is found to be finer, compared with that in unreinforced Al-Si alloy. The Al2O3 particles decomposed from the AACH (Fig.1(b)) distribute uniformly in the matrix and the particle size of γ-Al2O3 is measured to be 0.5-1.0 μm in diameter, although some of them are much smaller. Fig.1(c) indicates that the γ-Al2O3 particles agglutinate and distribute nonuniformly in the matrix.

Fig.1 SEM micrographs of matrix and composites: (a) Al-Si alloy matrix; (b) Composite by adding AACH; (c) Composite by adding γ-Al2O3
A differential thermal analysis (DTA) was carried out to confirm the onset temperature of the reactions of AACH in molten Al-Si alloy, as typically shown in Fig.2. The endothermic peak occurs at about 183 ℃ due to the following reaction:
2NH4AlO(OH)HCO3(s)→Al2O3(s)+2NH3(g)+2CO2(g)+3H2O(g)
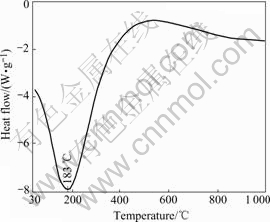
Fig.2 DTA curve of AACH powder heated at 10℃/min in Ar atmosphere
Fig.3 shows the X-ray diffraction (XRD) pattern of the specimen obtained from the method presented above. The chemical reaction takes place thoroughly, therefore γ-Al2O3 particles in the specimen are obtained and residual AACH is not found.
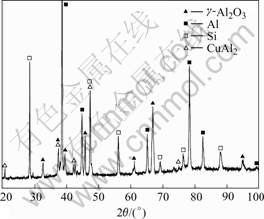
Fig.3 XRD pattern of composite fabricated by adding AACH
The used AACH is known to be one of the raw materials for fabricating commercial superfine high- purity Al2O3[14, 15]. The type of Al2O3 decomposed from the AACH is γ-Al2O3 with a larger specific surface area and a better sorption capacity[16]. At the same time, the Al2O3 particles are in spherical like shapes, which have better wettability with the molten aluminum, so they can bond firmly with the aluminum matrix. The microstructure of this kind of composites is able to reduce stress concentration and easy to transmit the load, so it can improve the properties of the composite.
3.2 Wear behavior
The dry sliding wear tests were carried out under a contact load of 20 N at a sliding velocity of 0.2 m/s. The variation in volume loss of unreinforced Al-Si matrix and two composites pin specimens as a function of sliding distance is shown in Fig.4. The volume loss of unreinforced Al-Si alloy is about 3 times larger than that of the two composites and that of the composite fabricated by adding AACH is smaller than that by adding γ-Al2O3.
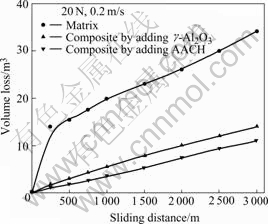
Fig.4 Volume loss of specimens as function of sliding distance (dry sliding)
Fig.5 shows the variation in volume loss of three specimens as a function of load after sliding for 2 000 m under lubricated condition. The volume loss of unreinforced Al-Si alloy is about 3 times larger than that of the two composites. With the increasing of load, the volume loss of the matrix increases rapidly, but slowly for the composites. The wear resistance of the composite is better than that of the corresponding alloy matrix mainly due to the presence of γ-Al2O3 particles for taking the external load.

Fig.5 Volume loss of specimens as function of load (lubricated sliding distance 2 000 m)
Based on Fig.4 and Fig.5, the volume loss of the composite prepared by adding AACH is smaller than that of the composite prepared by adding Al2O3, so the better wear resistance of the composite prepared by adding AACH is mainly due to the γ-Al2O3 particles decomposed from AACH distributed uniformly in the matrix.
4 Conclusions
1) An in situ γ-Al2O3 reinforced Al-Si alloy matrix composite was successfully fabricated by adding AACH, in which γ-Al2O3 particles distribute uniformly.
2) In the composites, the amount of flaky eutectic Si is lower and acicular eutectic Si is finer, compared with that in Al-Si alloy matrix.
3) The volume loss of unreinforced Al-Si alloy matrix is about 3 times larger than that of the composites and that of the composite fabricated by adding AACH is smaller than that by adding γ-Al2O3.
References
[1] WU J M, LI Z Z. Nanostructured composite obtained by mechanically driven reduction reaction of CuO and Al powder mixture [J]. Journal of Alloys and Compounds, 2000, 299(3): 9-16
[2] ZHU S J, IIZUKA T. Fabrication and mechanical behavior of Al matrix composites reinforced with porous ceramic of in situ grown whisker framework [J]. Mater Sci Eng A, 2003, 354: 306-313.
[3] HAL I W, BARRAILLE V. The effect of thermal exposure on the microstructure and fiber/matrix interface of an Al2O3/Al composite [J]. Metall Trans A, 1986, 17: 1075-1080.
[4] ZHONG Zhao-wei. Grinding of alumina/aluminum composites [J]. J Mater Proc Tech, 2002, 123: 13-18.
[5] WANG Hong, FU Gao-feng, JIANG Lan, SHI Yu-juan, LIU Chang-sheng. Study on in situ Al/Al2O3 composites by adding NH4Al(SO4)2 into pure Al melt [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(2): 310-314. (in Chinese)
[6] GUO R Q, ROHATGI P K. Chemical reactions between aluminum and fly ash during synthesis and reheating of Al-fly ash composite [J]. Metall Mater Trans B, 1998, 29: 519-525.
[7] WANG Y G, BRONSVELD P M. Twinning in θ-alumina investigated with high resolution transmission electron microscopy [J]. J Eur Ceram Soc, 1998, 18: 299-305.
[8] KOCZAK M J, PREMKUMAR M K. Emerging technologies for the in-situ production of MMCs [J]. JOM, 1993, 45: 44-48
[9] CHEN G, SUN G X, ZHU Z G. Study on reaction-processed Al-Cu/α-Al2O3(p) composites [J]. Mater Sci Eng A, 1999, 265: 197-201.
[10] FAN T X, ZHANG D, YANG G, TOSHIYA S, MASSAKI N. Fabrication of in situ Al2O3/Al composite via remelting [J]. J Mater Proc Tech, 2003, 142: 556-561.
[11] SUBRAMANIAN R, MCKAMEY C G, BUCK L R, SCHNEIBEL F H. Synthesis of iron aluminide-Al2O3 composites by in-situ displacement reactions [J]. Mater Sci Eng A, 1997, 239/240: 640-649.
[12] HUANG Zan-jun, YANG Bin, CUI Hua, ZHANG Ji-shan. Study on the fabrication of Al matrix composites strengthened by combined in-situ alumina particle and in-situ alloying elements [J]. Mater Sci Eng A, 2003, 351:15-22.
[13] CHEN G, SUN F X. Study on in situ reaction-processed Al-Zn/α-Al2O3(p) composites [J]. Mater Sci Eng A, 1998, 244: 291-295.
[14] LI Dong-hong, WIN Jiu-ba, LI Wang-xing. Study on preparation of nano-sized alumina by AACH thermal decomposition [J]. Journal of Henan University of Science and Technology, 2005, 26(4): 1-4. (in Chinese)
[15] FU Gao-feng, BI Shi-wen, YANG Yi-hong, LI Dian-feng. Preparation of super-fine α-Al2O3 by NH4AlO(OH)HCO3 thermal decomposition [J]. The Chinese Journal of Nonferrous Metals, 1998, 8(Suppl.2): 64-66. (in Chinese)
[16] SHEN Xiao-qing, LI Zhong-jun,YAO Hong-chang. Preparation of nanosized alumina powders by pyrolysis of ammonium aluminium carbonate hydroxide [J]. Chinese Journal of Inorganic Chemistry, 2003, 19(6): 651-654. (in Chinese).
Foundation item: Project(105055) supported by Key Project of Ministry of Education of China
Corresponding author: FU Gao-feng; Tel: +86-24-83681325; E-mail: fugf@smm.neu.edu.cn
(Edited by YUAN Sai-qian)