聚合羟基磷酸铁对土壤中镉和铅的固定
来源期刊:中国有色金属学报(英文版)2017年第5期
论文作者:袁艺宁 柴立元 杨志辉 吴瑞平 刘恢 梁丽芬 侍维
文章页码:1165 - 1171
关键词:土壤;镉;铅;聚合羟基磷酸铁;固定修复
Key words:soil; Cd; Pb; polymeric hydroxyl ferric phosphate; immobilization remediation
摘 要:利用二氧化钛生产过程中产生的富含硫酸亚铁的副产物在碱性条件下制备聚合羟基磷酸铁(PHFP),用于固定土壤中的铅和镉。利用傅里叶变换红外光谱和X射线衍射分析所制备聚合羟基磷酸铁的结构特征;采用改进的Tessier连续提取法并模拟酸雨淋溶以评价土壤中镉和铅的固定效果。结果表明,羟基磷酸铁是一种含有复杂OH—Fe—P结构的多聚物,主要由Fe6(OH)5(H2O)4(PO4)4(H2O)2 和 Fe25(PO4)14(OH)24组成。当其以4%的比例加入土壤中,土壤中DTPA提取态镉和铅的去除率分别达33%和45%,水溶态镉和铅的去除率分别降低了56%和58%。镉和铅的固定是由于水溶态、交换态和碳酸盐结合态转化为铁锰氧化物结合态、有机结合态和残渣态。模拟酸雨淋溶下,采用羟基磷酸铁修复后土壤中镉和铅的释放量较修复前分别下降了53%和52%。结果表明该羟基磷酸铁具有修复镉和铅污染土壤的应用潜力。
Abstract: A polymeric hydroxyl ferric phosphate (PHFP) was prepared by using a byproduct of titanium dioxide containing ferrous sulfate and phosphates under alkaline condition. The PHFP was used to immobilize lead (Pb) and cadmium (Cd) in soils. Fourier transform infrared spectra, X-ray diffraction were applied to revealing the characteristics of PHFP, and the modified Tessier sequential extraction and column leaching experiment with simulated acid rain were used to assess the effectiveness of immobilization of Cd and Pb in soils by PHFP. The results showed that PHFP was indeed a polymer with complicated OH—Fe—P structure and consisted of Fe6(OH)5(H2O)4(PO4)4(H2O)2 and Fe25(PO4)14(OH)24. Moreover, the removal rates of DTPA-extractable Cd and Pb in soils reached up to 33% and 45%, and the water-soluble Cd and Pb decreased by 56% and 58%, respectively, when PHFP was added in soils at 4% dosage. In addition, the immobilization of Cd and Pb contributed to transforming water soluble, exchangeable and carbonate-bonded fractions to Fe and Mn oxides-bonded, organic-bonded and residual fractions. Under leaching with simulated acid rain, Cd and Pb release amount in PHFP amended soil declined by 53% and 52%, respectively, as compared with non-treated soil. The result implied that PHFP had a potential application for the remediation of Cd- and Pb-contaminated soils.
Trans. Nonferrous Met. Soc. China 27(2017) 1165-1171
Yi-ning YUAN1,2, Li-yuan CHAI1,2, Zhi-hui YANG1,2, Rui-ping WU1,3, Hui LIU1,2, Li-fen LIANG1, Wei SHI1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China;
3. China BlueStar Changsha Design and Research Institute, Changsha 410116, China
Received 30 June 2016; accepted 11 January 2017
Abstract: A polymeric hydroxyl ferric phosphate (PHFP) was prepared by using a byproduct of titanium dioxide containing ferrous sulfate and phosphates under alkaline condition. The PHFP was used to immobilize lead (Pb) and cadmium (Cd) in soils. Fourier transform infrared spectra, X-ray diffraction were applied to revealing the characteristics of PHFP, and the modified Tessier sequential extraction and column leaching experiment with simulated acid rain were used to assess the effectiveness of immobilization of Cd and Pb in soils by PHFP. The results showed that PHFP was indeed a polymer with complicated OH—Fe—P structure and consisted of Fe6(OH)5(H2O)4(PO4)4(H2O)2 and Fe25(PO4)14(OH)24. Moreover, the removal rates of DTPA-extractable Cd and Pb in soils reached up to 33% and 45%, and the water-soluble Cd and Pb decreased by 56% and 58%, respectively, when PHFP was added in soils at 4% dosage. In addition, the immobilization of Cd and Pb contributed to transforming water soluble, exchangeable and carbonate-bonded fractions to Fe and Mn oxides-bonded, organic-bonded and residual fractions. Under leaching with simulated acid rain, Cd and Pb release amount in PHFP amended soil declined by 53% and 52%, respectively, as compared with non-treated soil. The result implied that PHFP had a potential application for the remediation of Cd- and Pb-contaminated soils.
Key words: soil; Cd; Pb; polymeric hydroxyl ferric phosphate; immobilization remediation
1 Introduction
Heavy metal contamination of soil is one of the most compelling global problems [1]. The heavy metals in contaminated soils result from mining and smelting of metal ores, mineral waste storage and discharge of industrial wastewater [2-12]. The accumulation of heavy metals in soil seriously affects the food security and human health. Heavy metals may enter the human body via the food chain, and then cause the potential hazards to human’s health as a consequence [13-15]. Therefore, it is urgent to develop technologies for the remediation of heavy metal-contaminated soils.
Generally, soil remediation technologies include physical remediation, chemical remediation, phyto- remediation and micro-remediation. Among various remediation technologies, in-situ chemical immobilization is less expensive and may provide a long-term remediation solution through generating low- solubility minerals or precipitates [16-25]. During the last two decades, many studies have been reported to evaluate the ability of different chemical amendments to immobilize Cd and Pb in polluted soils [26-28]. These amendments include clay minerals (kaolinite, mont- morillonite, sepiolite, zeolite), iron-containing materials (molysite, iron-bearing minerals, nanoscale zero-valent iron) [29,30], phosphates (hydroxyapatite, phosphate rocks, hydrogen phosphate) [31-34], and others (lime, activated carbon, manure, bone meal) [35-39].
Phosphate compounds and related materials have been proven to be effective agents for in situ immobilization of heavy metals in contaminated soils/sediments and solid wastes [33,40]. In the presence of phosphates in the soils, lead and phosphate can react quickly to generate precipitate of lead phosphate compound with low solubility, especially the pyromorphite [Pb5(PO4)3(Cl, OH, F)] [33]. The toxicity of Pb is weakened through reducing its chemical mobility [41]. Likewise, copper phosphates and cadmium phosphates also have fairly lower solubility than other forms of compounds. Adsorption behavior of cadmiun onto the surface of iron oxide minerals confirmed that the adsorption of Cd onto α-FeOOH, β-FeOOH and Fe8O8(OH)6SO4 was specific adsorption [42]. The absorption of As(III) by zero-valent iron was mainly due to the oxidation by Fe(0) (magnetic iron, magnetic hematite and lepidocrocite) [43]. Thus, —OH group- containing material can effectively immobilize heavy metals in soils because of adsorption, in particular, specific adsorption.
This study presumes the feasibility of using polymeric hydroxyl ferric phosphate (PHFP) as a novel immobilizer for in situ immobilization of Cd(II) and Pb(II) in soils since PHFP contains both phosphate and —OH group. Therefore, PHFP was synthesized using iron vitriol (the byproduct in titanium pigment production), phosphate, oxidant and alkaline solution. The morphology characteristics and chemical constitution of PHFP were studied by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy and X-ray diffraction (XRD). Batch experiments were further carried out to evaluate the remediation efficiencies of Cd and Pb in soils by using PHFP.
2 Experimental
2.1 Soil sampling
The soil sample of the upper layer (0-20 cm) was collected in the vicinity of lead-zinc smelter in Hunan Province, China (113°17′22″E, 25°45′40″N). The moist soil was air-dried and ground to pass through a 1 mm- sieve. Soil was homogeneously spiked with Cd and Pb by spraying with aqueous solutions of Cd(NO3)2 and Pb(NO3)2 and aged for 40 d. The chemical characteristics of the contaminated soil were: total Pb 2506 mg/kg, total Cd 18.6 mg/kg, DTPA extractable Pb 2303 mg/kg, DTPA extractable Cd 10.0 mg/kg, water soluble Pb 20.2 mg/kg, water soluble Cd 6.8 mg/kg and pH (H2O) 3.61.
2.2 Preparation of PHFP
45.36 g of byproducts of titanium dioxide containing 75% (mass fraction) of FeSO4·7H2O was dissolved in 300 mL of deionized water, and mixed with Ca(H2PO4)2 under stirring based on 0.6:1 of molar ratio of P to Fe. Hydrogen peroxide solution was dropped into the mixed solution according to 1.25:1 of molar ratio of H2O2 to Fe. Meanwhile, the carbide slag solution was added into the mixed solution to adjust pH value to 7.0 under vigorously stirring. Finally, the mixture was reacted for 2 h in a thermostatic water bath of 80 °C and then kept at room temperature for 24 h to obtain the stabilized reddish brown precipitate. The precipitate was recovered by vacuum filtration followed by washing with water and drying at 70 °C.
2.3 Characterization of PHFP
The structure of PHFP was characterized by XRD (D/max 2550 VB+X) with a Cu Kα irradiation over a range of 5°<2θ<70°. The morphology of PHFP was observed by SEM (Zeiss Gemini 1530 FEG) and TEM (TECNAI20). A FT-IR spectrum of PHFP was recorded using a FT-IR spectrometer (NICOLET IS10). The sample was scanned under 400 to 4000 cm-1 at 4 cm-1 resolution. The specific surface area of PHFP was determined by Brunauer-Em-mett-Teller (BET) method using a micrometrics instrument (NOVA-1000).
2.4 Immobilization test of Cd and Pb in soils
0.5, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0 g PHFP were thoroughly mixed with 100 g soils, respectively. The deionized water was added into the mixture to adjust soil moisture to 80% of water holding capacity. The mixture was placed for 60 d at room temperature. Then, the mixture was withdrawn to analyze the concentrations of water soluble and DTPA-extractable Cd and Pb. All the treatments were performed in triplicate. The non-treated soil was used as a control treatment.
2.5 Sequential extraction of Cd and Pb fractions in soils
The fractionation of Cd and Pb in soils was determined using a modified Tessier sequential extraction method [44,45]. Briefly, six fractions of Cd and Pb were separated by extracting with distilled water (water soluble fraction), extracting with 1 mol/L MgCl2 at pH 7 (exchangeable fraction), extracting with 1 mol/L NaOAc at pH 5 (carbonate-bonded fraction), extracting with 0.04 mol/L NH2OH·HCl in 25% (volume fraction) HOAc (Fe-Mn oxides-bonded fraction), extracting with 30% H2O2 in 0.02 mol/L HNO3 at pH 2 (organics-bonded fraction), and extracting with HClO4 and HF (residual fraction). Between each successive extraction, separation was employed by centrifuging at 12000 r/min for 20 min. The supernatant was collected and the concentrations of Cd and Pb in supernatant were measured using an inductively coupled plasma-optical emission spectrometer (ICP-OES) (Optima 5300 DV). All the experiments were carried out in triplicates.
2.6 Soil column leaching experiment with simulated acid rain
To investigate the performance of the soil treated by PHFP in the long term, soil samples were artificially leached with simulated acid rain. Simulated acid rain was designed according to the main ion composition and pH of local rainfall [26]. At the sampling site, the pH of rainfall varied from 3.8 to 4.9. Therefore, the simulated acid rain of pH 4.0 was prepared with 3:1 (volume ratio) H2SO4:HNO3 solution. The concentrations of  ,
,  , NH4+, Ca2+, Mg2+, K+, Na+ and Cl- were 73.65, 23.39, 18.71, 64.25, 6.99, 6.14, 6.14 and 20.02 mmol/L, respectively.
, NH4+, Ca2+, Mg2+, K+, Na+ and Cl- were 73.65, 23.39, 18.71, 64.25, 6.99, 6.14, 6.14 and 20.02 mmol/L, respectively.
Column leaching experiment was performed in a plexiglass column with 50 cm in height and 12 cm in interior diameter. Non-treated and PHFP-treated soils were used for the simulated rain leaching experiment. 1 kg air-dried soil, passed through a 0.2 mm sieve, was filled into a plexiglass column at a bulk density of 1.3 g/cm3. The average annual rainfall is around 1410 mm at the location where the soil samples were collected. Then 30% of runoff was deducted. Thus, the volume of the simulated rain was 750 mL per year (one cycle). A total of 7500 mL simulated rain solution with 10 times (10 cycles) was slowly sprayed from the top of the column at a rate of 32 mL/h controlled by a peristaltic pump. The leachate was collected from the column bottom. The concentrations of Cd and Pb in leachate were determined by using ICP-OES (Optima 5300 DV).
2.7 Chemical measurement
The water soluble and DTPA extractable Cd and Pb were extracted by deionized water and 0.5 mol/L DTPA solution at soil/water ratio of 1:5 (m:V). Total Cd and Pb in soils were digested with HClO4 and HF. The concentrations of Cd and Pb in solution of water extraction, DTPA extraction, and digested solution were measured by using ICP-OES (Optima 5300 DV).
3 Results and discussion
3.1 Characterization of PHFP
The morphology and chemical constitution of PHFP obtained under different conditions were investigated. As shown in TEM images (Fig. 1), PHFP had a smooth surface and primarily regular tubular structure with the lengths of 2-5 μm and diameters of 80-200 nm. Besides the columnar shapes, a small amount of flakes or particulates were also observed,which might be due to the smaller diameter of PHFP.
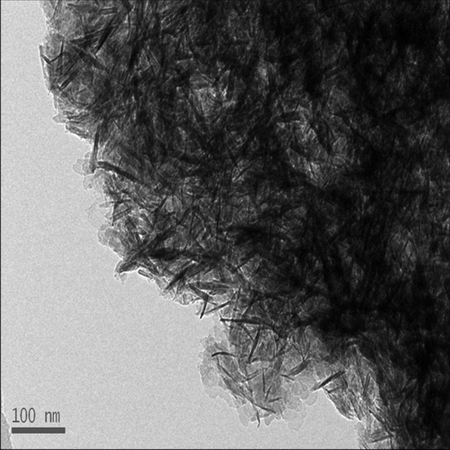
Fig. 1 TEM images of PHFP

Fig. 2 SEM images of PHFP
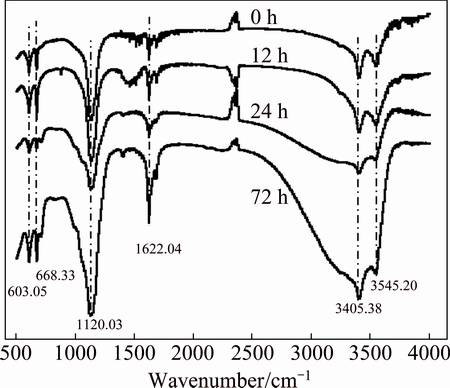
Fig. 3 FT-IR spectra of PHFP with different aging time
The SEM images of the PHFP are shown in Fig. 2. It can be clearly seen that PHFP had obvious agglomeration. In addition, based on BET result, the specific surface area of PHFP was 6.47 m2/g.
Figure 3 shows FT-IR spectra of PHFP at different aging time. The bands at 3405.38 cm-1 and 3545.20 cm-1 were broadened owing to stretching vibration of —OH in adsorbed water molecules and the —OH connected with the iron ion. While the absorption peak at 1622 cm-1 revealed the presence of flexural vibration mode (H—O—H in bound water molecules). The obvious and sharp bands at 900-1200 cm-1 were the scope of absorption peak of  including the band at 1120 cm-1 which represented the opposition stretching vibration of
including the band at 1120 cm-1 which represented the opposition stretching vibration of 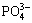 . Meanwhile, the weak absorption peaks at 600-700 cm-1 showed the vibration of the —Fe—O— bonds. Based on the morphology observation and functional groups, it can be concluded that PHFP was an amorphous and high polymer with complicated hydroxyl groups and iron that may be chelated with phosphorus, such as —Fe—O—Fe—, —P—O—P—, —Fe—P—Fe—, and —P—Fe—P—. Therefore, structure of PHFP can be expressed as repetitions of —{—P—O—[…O—H…]n—O—Fe—}—.
. Meanwhile, the weak absorption peaks at 600-700 cm-1 showed the vibration of the —Fe—O— bonds. Based on the morphology observation and functional groups, it can be concluded that PHFP was an amorphous and high polymer with complicated hydroxyl groups and iron that may be chelated with phosphorus, such as —Fe—O—Fe—, —P—O—P—, —Fe—P—Fe—, and —P—Fe—P—. Therefore, structure of PHFP can be expressed as repetitions of —{—P—O—[…O—H…]n—O—Fe—}—.
Furthermore, the aging time had a significant influence on the peak shape of FT-IR spectra (Fig. 3), and it illustrated the continuance of polymerization, which implied that the oligomeric state of Fe was continually transformed into the high poly Fe state.
The XRD pattern of PHFP is shown in Fig. 4. The results showed that PHFP predominantly consisted of Fe6(OH)5(H2O)4(PO4)4(H2O)2 and Fe25(PO4)14(OH)24. Meanwhile, Ca(FeFe)(OH)(PO4)2, Ca(Fe6(OH)6(H2O)2- (PO4)4)2 and Fe12(OH)7.3(PO4)8(H2O)4.7 were also observed in PHFP. The results revealed that the polymerisation occurred in PHFP, which was consistent with the results of FT-IR spectra (Fig. 3).

Fig. 4 XRD pattern of PHFP
3.2 Cd and Pb immobilization by PHFP
The PHFP was added into soils at seven levels (0.5%, 1%, 2%, 3%, 4%, 6%, 8% of soil mass) to determine the optimal dosage for Cd and Pb immobilization. As shown in Fig. 5, PHFP dosage had a significant influence on the removal efficiency of Cd and Pb in the polluted soil.
Obviously, the removal percentages of DTPA- extractable Cd and Pb significantly increased with the dosage increasing from 0.5% to 8%. When 4% of PHFP was applied, the removal rates of DTPA extractable Cd and Pb were achieved by 33% and 45%, respectively. In addition, the removal rates of water-soluble Cd and Pb also reached up to 56% and 58%, respectively. However, when PHFP dosage was above 4%, the removal rates of water-soluble Cd and Pb were tended to flatten out, although there was a slight increase. This might because that soil pH was elevated by the increasing PHFP dosage. The relatively high pH could reduce the adsorption ability of heavy metals. Therefore, considering the cost of PHFP, the optimal PHFP dosage was 4%.
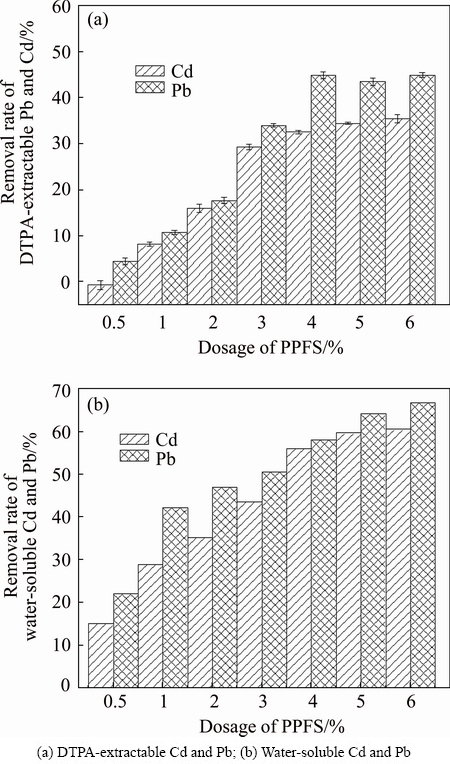
Fig. 5 Effect of PHFP dosage on removal of Cd and Pb
3.3 Changes of Cd and Pb fractions in soils after PHFP remediation
In the originally contaminated (non-treated) soil, the water soluble, exchangeable and carbonate-bonded Cd fractions accounted for 80% of the total Cd (Fig. 6), indicating that Cd had a high bioavailability. In this study, the soils showed a low pH value of 3.81. Under acidic condition, Cd could be susceptible to be converted from the stable fraction (such as Fe and Mn oxides- bonded, organics-bonded and residual fractions) to active fractions including water-soluble and exchangeable fractions. After PHFP amendment, the concentration of water-soluble, exchangeable and carbonate-bonded Cd fractions significantly decreased. Their corresponding values declined from 7.94, 4.09 and 2.93 mg/kg to 5.98, 1.97 and 1.87 mg/kg, respectively. Conversely, concentrations of Fe and Mn oxides-bonded, organics-bonded and residual Cd fractions increased. In particular, residual Cd increased by 20% as compared to the non-treated soils. Similar change trends were found in Pb fractions. The results indicated that Cd and Pb were associated with PHFP resulting in the decrease of reactive Cd and Pb fractions. Therefore, PHFP amendment can enhance the formation of stable Cd and Pb fractions and decrease Cd and Pb bioavailability.

Fig. 6 Distribution of Cd and Pb fractions in non-treated and PHFP-treated soils
3.4 Release of Cd and Pb from soils after PHFP remediation
In order to evaluate the stabilization of Cd and Pb in soils after PHFP remediation, soils with and without PHFP amendment were artificially leached with simulated acid rain. Figure 7 shows the accumulated release amount of Cd and Pb in soils under leaching 10 times (cycles) with the simulated acid rain. In general, accumulated release amount of Cd and Pb increased with increasing leaching cycle of the simulated acid rain and reached a maximum amount of Cd and Pb at the 4th leaching cycle and the 3rd leaching cycle, respectively. Before the 3rd leaching cycle, Cd and Pb were more easily leached out. Probably, the released Cd and Pb contributed to the release from the electrostatic adsored form. After the 5th leaching cycle, concentrations of Cd and Pb in leachate were not detected. In the non-treated soil, the maximum release amount of Cd and Pb amounted to 3.67 mg/kg and 16.30 mg/kg after the 10th leaching cycle, accounting for 19.47% and 0.65% of total Cd and Pb in soils. As compared with the non-treated soil, Cd and Pb release amount in PHFP amended soil declined by 53% and 52%, respectively. During leaching process, the simulated acid rain resulted in a decrease in soil pH. As a result, H+ in acid rain caused the dissolution of Fe and Mn oxides and resulted in the release of one portion of Cd and Pb bonded to Fe and Mn oxides. However, the release of Cd was easier than Pb (Fig. 7). This contributes to that Cd is more active than Pb. As compared with non-treated soil, PHFP remediation decreased the release of Cd and Pb from soils. Therefore, PHFP was effective for immobilization of Cd and Pb, reducing the potential risk of Cd and Pb contamination in soils.
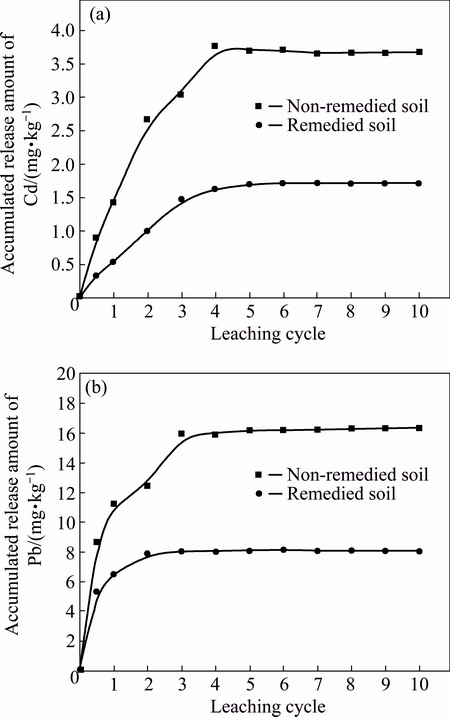
Fig. 7 Accumulated release amount of Cd (a) and Pb (b) from soils under leaching with simulated acid rain
4 Conclusions
1) PHFP was synthesized by using ferrous sulfate, phosphates, oxidizing agent and calcium carbide slag solution. PHFP was a polymer with a complicated OH—Fe—P structure and mainly consisted of Fe6(OH)5(H2O)4(PO4)4(H2O)2 and Fe25(PO4)14(OH)24.
2) When PHFP was added in soils at 4% dosage, the removal rate of DTPA-extractable Cd and Pb reached up to 33% and 45%, and the water-soluble Cd and Pb decreased by 56% and 58%, respectively. The addition of PHFP amendment resulted in decreasing dissolution of Cd and Pb, and a lower portion of Cd and Pb of water- soluble, exchangeable and carbonate-bonded fractions, decreasing mobility of Cd and Pb. The immobilization of Cd and Pb contributes to transforming their readily active fractions to stable fractions.
3) Under leaching with simulated acid rain, Cd and Pb release amount in PHFP amended soil declined by 53% and 52%, respectively, as compared with the non-treated soil. The result suggests that PHFP has a potential application for the remediation of Cd- and Pb-contaminated soils.
References
[1] WANG Zhen-xing, CHAI Li-yuan, YANG Zhi-hui, WANG Yun-yan, WANG Hai-ying. Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, Changsha, China [J]. Journal of Environmental Quality, 2010, 39: 1616-1623.
[2] TANG J W, LIAO Y P, YANG Z H, CHAI L Y, YANG W C. Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China [J]. Journal of Soils and Sediments, 2016, 16(5): 1519-1528.
[3] SUN Hong-fei, LI Yong-hua, JI Yan-fang, YANG Lin-sheng, WANG Wu-yi, LI Hai-rong. Environmental contamination and health hazard of lead and cadmium around Chatian mercury mining deposit in western Hunan Province, China [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 308-314.
[4] WANG Zhen-xing, CHAI Li-yuan, YANG Zhi-hui, WANG Yun-yan, WANG Hai-ying. Identifying sources and assessing potential risk of heavy metals in soils from direct exposure to children in a mine-impacted city, Changsha, China [J]. Journal of Environmental Quality, 2010, 39: 1616-1623.
[5] CHAI L Y, MUBARAK H, YAN Z H, YONG W, TANG C J, MIRZA N. Growth, photosynthesis, and defense mechanism of antimony (Sb)-contaminated Boehmeria nivea L [J]. Environmental Science and Pollution Research, 2016, 23(8): 7470-7481.
[6] YANG Sheng-xiang, LIAO Bin, YANG Zhi-hui, CHAI Li-yuan, LI Jin-tian. Revegetation of extremely acid mine soils based on aided phytostabilization: A case study from southern China [J]. The Science of the Total Environment, 2016, 562: 427-34.
[7] WANG Ai-yun, WANG Min-yan, LIAO Qi, HE Xi-quan. Characterization of Cd translocation and accumulation in 19 maize cultivars grown on Cd-contaminated soil: Implication of maize cultivar selection for minimal risk to human health and for phytoremediation [J]. Environmental Science and Pollution Research, 2016, 23(6): 5410-5419.
[8] WANG Y, CHAI L Y, YANG Z H,HUSSANI M, XIAO R Y,TANG C J.Subcellular distribution and chemical forms of antimony inFicus tikoua [J]. International Journal of Phytoremediation, 2017, 19(2): 97-103.
[9] WANG Y, CHAI L Y, YANG Z H, HUSSANI M, TANG C J. Chlorophyll fluorescence in leaves of Ficus tikoua under arsenic stress [J]. Bulletin of Environmental Contamination and Toxicology, 2016, 97(4): 576-581.
[10] MUBARAK H, CHAI L Y, MIRZA N, YANG Z H, PERVEZ A, TARIQ M, SHAHEEN S, MAHMOOD Q. Antimony (Sb)– pollution and removal techniques–critical assessment of technologies [J]. Toxicological & Environmental Chemistry, 2015, 97(10): 1296-1318.
[11] YANG Sheng-xiang, CAO Jian-bing, LI Feng-mei, PENG Xi-zhu, PENG Qing-jing, YANG Zhi-hui, CHAI Li-yuan. Field evaluation of the effectiveness of three industrial by-products as organic amendments for phytostabilization of a Pb/Zn mine tailings [J]. Environmental Science-Processes & Impacts, 2016,18(1): 95-103.
[12] MA Li, SUN Jing, YANG Zhao-guang, WANG Lin. Heavy metal contamination of agricultural soils affected by mining activities around the Ganxi River in Chenzhou, Southern China [J]. Environmental Monitoring and Assessment, 2015, 187: 730-738.
[13] WANG Yang-yang, CHAI Li-yuan, LIAO Qi, TANG Chong-jian, LIAO Ying-ping, PENG Bing, YANG Zhi-hui. Structural and genetic diversity of hexavalent chromium-resistant bacteria in contaminated soil [J]. Geomicrobiology Journal, 2016, 33(3-4): 222-229.
[14] YANG Z H, ZHANG Z, CHAI L Y, WANG Y, LIU Y, XIAO R Y. Bioleaching remediation of heavy metal-contaminated soils using Burkholderia sp. Z-90 [J]. Journal of Hazardous Materials, 2016, 301: 145-152.
[15] DAI Hai-jiang, HUANG Zhi-jun, DENG Qi-hong, LI Ying, XIAO Ting, NING Xing-ping, LU Yao, YUAN Hong. The effects of lead exposure on serum uric acid and hyperuricemia in Chinese adults: A cross-sectional study [J]. International Journal of Environmental Research and Public Health, 2015, 12: 9672-9682.
[16] YANG M, XIAO X Y, MIAO X F, GUO Z H, WANG F Y. Effect of amendments on growth and metal uptake of giant reed (Arundo donax L.) grown on soil contaminated by arsenic, cadmium and lead [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(6): 1462-1469.
[17] LEE S H, LEE J S, CHOI Y J, KIM J G. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments [J]. Chemosphere, 2009, 77(8): 1069-1075.
[18] CHAI L Y, HUANG S H, YANG Z H, PENG B, HUANG Y, CHEN Y H. Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soils under chromium-containing slag heap [J]. Journal of Environmental Science and Health (Part A), 2009, 44 (6): 615-622.
[19] CHAI Li-yuan, WANG Yong, YANG Zhi-hui, MUBARAK H, MIRZA N. Physiological characteristics of Ficus-tikoua under antimony stress [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(4): 939-945.
[20] TANG Hui, LIU Yun-guo, GONG Xiao-min, ZENG Guang-ming, ZHENG Bo-hong, WANG Da-fei, SUN Zhi-chao, ZHOU Lu, ZENG Xiao-xia. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress [J]. Environmental Science and Pollution Research, 2016, 23(8): 7470-7481.
[21] WANG Z X, PANG Z H, GUO Q W, CHEN J Y, XU Z C, LEI Y T, CHEN J Q, SUN G Q, HU X B, LUO Q J. Introducing a land-use-based spatial analysis method for human health risk evaluation of soil heavy metals [J]. Environmental Earth Sciences, 2013, 70(7): 3225-3235.
[22] WU Chuan, ZOU Qi, XUE Sheng-guo, MO Jing-yu, PAN Wei-song, LOU Lai-qing, WONG Ming-hung. Effects of silicon (Si) on arsenic (As) accumulation and speciation in rice (Oryza sativa L.) genotypes with different radial oxygen loss (ROL) [J]. Environmental Pollution, 2016: 12: 27-33.
[23] YANG Zhi-hui, LIU Lin, CHAI Li-yuan, LIAO Ying-ping, YAO Wen-bin, XIAO Rui-yang. Arsenic immobilization in the contaminated soil using poorly crystalline Fe-oxyhydroxy sulfate [J]. Environmental Science and Pollution Research, 2015, 22: 12624-12632.
[24] GUO Z H, HE Z X, ZHOU K G. Growth response and tolerance of Job's tears (Coix lacryma-jobi L) to arsenic in spiked soils [J]. International Journal of Environment & Pollution, 2009, 37(2-3): 133-140.
[25] LIAO Ying-ping, MIN Xiao-bo, YANG Zhi-hui, CHAI Li-yuan, ZHANG Shu-juan, WANG Yang-yang. Physicochemical and biological quality of soil in hexavalent chromium-contaminated soils as affected by chemical and microbial remediation [J]. Environmental Science and Pollution Research, 2014, 21: 379-388.
[26] ALVAREZ-AYUSO E, GARCIA-SANCHEZ A. Sepiolite as a feasible soil additive for the immobilization of cadmium and zinc [J]. The Science of the Total Environment, 2003, 305(1-3): 1-12.
[27] KOMAREK M, VANEK A, ETTLER V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review [J]. Environmental Pollution, 2013, 172: 9-22.
[28] ZHANG Shu-juan, YANG Zhi-hui, WU Bao-lin, WANG Yang-yang, WU Rui-ping, LIAO Ying-ping. Removal of Cd and Pb in calcareous soils by using Na2EDTA recycling washing [J]. Clean- Soil Air Water, 2014, 42(5): 641-647.
[29] YUAN C, LIEN H. Removal of arsenate from aqueous solution using nanoscale iron particles [J]. Water Quality Research Journal of Canada, 2006, 41: 210-215.
[30] KIM K, LEE B, KIM K. Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating [J]. Journal of Geochemical Exploration, 2012, 113: 124-129.
[31] MAVROPOULOS E, ROSSI A M, COSTA A M, PEREZ C A, MOREIRA J C, SALDANHA M. Studies on the mechanisms of lead immobilization by hydroxyapatite [J]. Environmental Science & Technology, 2002, 36(7): 1625-1629.
[32] YOON J K, CAO X, MA L Q. Application methods affect phosphorus-induced lead immobilization from a contaminated soil [J]. Journal of Environmental Quality, 2007, 36(2): 373-378.
[33] LIU R, ZHAO D. In situ immobilization of Cu(II) in soils using a new class of iron phosphate nanoparticles [J]. Chemosphere, 2007, 68(10): 1867-1876.
[34] MIRETZKY P, FERNANDEZ-CIRELLI A. Phosphates for Pb immobilization in soils: A review [J]. Environmental Chemistry Letters, 2008, 6: 121-133.
[35] MUEHE E M, ADAKTYLOU I J, OBST M, ZEITVOGEL F, BEHRENS S, PLANER-FRIEDRICH B, KRAEMER U, KAPPLER A. Organic carbon and reducing conditions lead to cadmium immobilization by secondary Fe mineral formation in a pH-neutral soil [J]. Environmental Science & Technology, 2013, 47(23): 13430-13439.
[36] GRAY C W, DUNHAM S J, DENNIS P G, ZHAO F J, MCGRATH S P. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud [J]. Environmental Pollution, 2006, 142(3): 530-539.
[37] CAO X, MA L, LIANG Y, GAO B, HARRIS W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar [J]. Environmental Science & Technology, 2011, 45(11): 4884-4889.
[38] TSANG D C, YIP A C, OLDS W E, WEBER P A. Arsenic and copper stabilisation in a contaminated soil by coal fly ash and green waste compost [J]. Environmental Science and Pollution Research, 2014, 21(17): 10194-10204.
[39] WANG Yun-yan, YAO Wen-bin, WANG Qing-wei, YANG Zhi-hui, LIANG Li-feng, CHAI, Li-yuan. Synthesis of phosphate-embedded calcium alginate beads for Pb(II) and Cd(II) sorption and immobilization [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(8): 2230-2237.
[40] CHAI L Y, TANG J W, LIAO Y P, YANG Z H, LIANG L F, LI Q Z, WANG H Y, YANG W C. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans and its application in arsenic immobilization in the contaminated soil [J]. Journal of Soils and Sediments, 2016, 16(10): 2430-2438.
[41] CLEMENS S C W H. Trace metal stabilization in a shooting range soil: mobility and phytotoxicity [J]. Journal of Hazardous Materials, 2007, 141: 378-387.
[42] KOVA EVI D, POHLMEIER A, ZBA G, NARRES H D, KALLAY M J N. The adsorption of lead species on goethite [J]. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 2000, 166(1-3): 225-233.
[43] RAJ K S, BRUCE M, LAURENT C, HEECHUL C. Removal of arsenic(III) from groundwater by nano scale zero-valent iron [J]. Environmental Science & Technology, 2005, 39: 1291-1298.
[44] TESSIER A, CAMPBELL P, BISSON M. Sequential extraction procedure for the speciation of particulate trace metals [J]. Analytical Chemistry, 1979, 51: 844-851.
[45] LIAO Y P, YANG Z H, CHAI L Y, WANG Z X. Migration and transfer of chromium in soil-vegetable system and associated health risks in vicinity of ferro-alloy manufactory [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(11): 2520-2527.
袁艺宁1,2,柴立元1,2,杨志辉1,2,吴瑞平1,3,刘 恢1,2,梁丽芬1,侍 维1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083;
3. 化工部长沙设计研究院,长沙 410116
摘 要:利用二氧化钛生产过程中产生的富含硫酸亚铁的副产物在碱性条件下制备聚合羟基磷酸铁(PHFP),用于固定土壤中的铅和镉。利用傅里叶变换红外光谱和X射线衍射分析所制备聚合羟基磷酸铁的结构特征;采用改进的Tessier连续提取法并模拟酸雨淋溶以评价土壤中镉和铅的固定效果。结果表明,羟基磷酸铁是一种含有复杂OH—Fe—P结构的多聚物,主要由Fe6(OH)5(H2O)4(PO4)4(H2O)2 和 Fe25(PO4)14(OH)24组成。当其以4%的比例加入土壤中,土壤中DTPA提取态镉和铅的去除率分别达33%和45%,水溶态镉和铅的去除率分别降低了56%和58%。镉和铅的固定是由于水溶态、交换态和碳酸盐结合态转化为铁锰氧化物结合态、有机结合态和残渣态。模拟酸雨淋溶下,采用羟基磷酸铁修复后土壤中镉和铅的释放量较修复前分别下降了53%和52%。结果表明该羟基磷酸铁具有修复镉和铅污染土壤的应用潜力。
关键词:土壤;镉;铅;聚合羟基磷酸铁;固定修复
(Edited by Xiang-qun LI)
Foundation item: Project (2012GS430203) supported by Science and Technology Program for Public Wellbeing, China; Project (51504299) supported by the National Natural Science Foundation of China; Project (2015WK3016) supported by Science and Technology Program of Hunan Province, China
Corresponding author: Zhi-hui YANG; Tel: +86-731-88836804; Fax: +86-731-88710171; E-mail: yangzh@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60136-5

