


Trans. Nonferrous Met. Soc. China 22(2012) 1661-1666
Surface modification of NiTi alloy by sol-gel derived porous TiO2 film
FU Tao1,2, WU Xiao-ming1, WU Feng3, LUO Meng1, DONG Bing-hui1, JI Yuan1
1. Key Laboratory of Biomedical Information Engineering of Ministry of Education,
School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, China;
2. State Key Laboratory of Metastable Materials Science and Technology,Yanshan University, Qinhuangdao 066004, China;
3. College of Medicine, Xi’an Jiaotong University, Xi’an 710061, China
Received 27 August 2011; accepted 30 September 2011
Abstract: Titania films with nano-sized pores were prepared on the NaOH-HCl pretreated NiTi alloy substrate by sol-gel method. A crack-free film is obtained for the sample with a dense inner layer and a porous outside layer (sample TC1+1). The X-ray diffraction shows that the titania films are composed of anatase, and a little Ni4Ti3 phase in the heat treated substrate is also detected. The X-ray photoelectron spectroscopy results indicate that the titania film completely covered the NiTi substrate for sample TC1+1. The sample TC1+1 is hydrophilic with a contact angle about 20°, and UV illumination treatment for 15 min further decreases the contact angle to (9.2±3.2)°. The potentiodynamic polarization test in 0.9% NaCl solution reveals a better corrosion resistance of sample TC1+1 than the polished NiTi sample.
Key words: NiTi alloy; titania; sol-gel method; hydrophilicity; corrosion resistance
1 Introduction
NiTi alloy has potentially wide biomedical applications especially in orthopedic, cardiovascular and dental implantations by virtue of its unique shape memory effect, superelasticity and high damping capacity [1]. However, corrosion of the nearly equiatomic NiTi alloy and possible release of toxic and carcinogenetic Ni ions are concerned problems for NiTi implants that will service in human body for a long period of time. Therefore, it is essential to improve the corrosion resistance and biocompatibility of NiTi implants by surface modification.
Titania coatings are demonstrated to be a corrosion resistant and well biocompatible material for surface modification of titanium, NiTi and other alloys [2-9]. Among the coating techniques, sol-gel method has advantages of the independence of substrate shape and good control of coating composition, thickness and topography [2,5,8]. In the previous work [7], a technical method of forming porous surface layer by the NaOH-HCl treatment was used to improve adhesion and integrity of the sol-gel TiO2 film on NiTi alloy.
Recently, the special wetting behaviors of titania films have been intensively researched due to their scientific interests and potential applications in different fields. For biomaterials, wettability and surface energy play an important role in biological performance of implant materials [10-14]. The hydrophilic surface can improve bioactivity and bone-bonding behavior of titanium [10,11], and reduce platelet adhesion, and in turn minimizes thrombogenic behavior of materials for cardiovascular implants [14]. Studies have shown that titania films consisting of nanostructures, e.g. nanotubes [15,16], nanosheets [17], nanorods [18,19], etc, exhibit high- or super-hydrophilic behaviors, with or without the ultraviolet (UV) illumination treatment.
In this work, porous titania films on NiTi alloy are prepared by using TiO2 sol containing polyethylene glycol (PEG), and the NaOH-HCl pretreatment is also used to improve adhesion and integrity of the porous titania films. Hydrophilicity and corrosion resistance of the titania coated NiTi alloy are investigated also.
2 Experimental
Nearly equiatomic hot rolled NiTi alloy plate (50.7% Ni, mole fraction, from Xi’an Saite Metal Materials Development Co., Ltd), with thickness of 2 mm, was sparkle cut into pieces of 10 mm ? 10 mm. The small plates were polished with SiC papers down to grits 1200, ultrasonically cleaned in acetone, ethanol and deionized (DI) water, and dried in air. The plates were then treated in 2.5 mol/L NaOH solution at 60 ℃ for 24 h. The alkali treated NiTi samples were rinsed in DI water several times, and soaked in dilute HCl solution at room temperature for 12 h. The evacuated samples were rinsed in DI water again and dried for sol-gel coating.
Precursor solution for TiO2 coating was prepared by the following method [7,20]. Tetrabutylorthotitanate (17.02 g) and diethanolamine (5.26 g) were dissolved in ethanol (30.52 g). The solution was stirred vigorously for 30 min at room temperature, and the mixture of water (0.90 g) and ethanol (30.52 g) was slowly added. The molar ratio of water to alkoxide was 1 in the solution. Part of the original solution was added with PEG 2000 with mass fraction of 2% to prepare PEG-containing solution. The TiO2 coating on the NiTi plates was prepared by dip-coating method at a withdrawal speed of 2 mm/s. The gel coating was dried at 105 ℃ for 5 min. The dipping-drying process was repeated up to 4 cycles using the above solutions to increase the coating thickness. The coating samples were then put directly into two hot electric furnaces sequentially for heat treatment at 400 ℃ for 10 min and at 500 ℃ for 10 min. The obtained coating samples with dip-coating layer number m, n using the original solution and the PEG-containing solution, respectively (m+n=1-4) were noted as PCm+n and TCm+n, respectively for the polished and NaOH-HCl treated NiTi plates.
Surface morphology of the samples was observed by scanning electron microscopy (SEM, FEI Quanta 600F), and crystallography structure was analyzed by X-ray diffraction (XRD, Cu Ka, Rigaku D/MAX-2400). Chemical bonding state and element composition of the samples were examined by X-ray photoelectron spectroscopy (XPS, Al Ka, K-Alpha, VG). The spectra were calibrated relatively to the C 1s peak (Eb=284.6 eV) resulting from the adventitious hydrocarbon present on the sample surface.
Contact angle of the NiTi samples was measured by injecting 5 mL DI water on the sample surface with a contact angle goniometer (JY-82, Dingsheng Test Machines Co. Ltd., China) under ambient condition. Some samples were subjected to UV illumination treatment (wave length centered at 253 nm) to increase hydrophilicity. Corrosion resistance of the samples was evaluated by potentiodynamic polarization tests in 0.9% NaCl solution (mass fraction) using an electrochemical workstation (Model CS150, Corrtest?) at ambient condition. A platinum electrode was used as the counter electrode, and the saturated calomel electrode (SCE) was the reference electrode.
3 Results and discussion
Figure 1 shows the SEM images of the prepared samples. The surface of sample PC1+0 is smooth and crack-free, but the porous film shrinks and a large area of the substrate is exposed for sample PC0+1 (Figs. 1(a), (b)). It seems that the lateral shrinkage is more serious for the PEG-containing gel film during the drying and heat treatment process. In order to balance volume shrinkage of the titania film during preparation, NiTi alloy surface was roughened by the NaOH-HCl treatment before dip coating (Fig. 1(c)). The formed porous surface layer can act as an interlayer to improve adhesion and integrity of the sol-gel titania film on NiTi substrate [7].
The XPS analysis indicates that Na element is not detectable, and the mole ratio of Ni to Ti is reduced to 0.14 on top surface of the NaOH-HCl treated NiTi sample. Thus, for the TC samples, one or two dense titania layers were coated so as to protect the alloy substrate and provide a barrier against Ni ion release before the coating of porous titania layer. For sample TC1+1, the porous morphology in Fig.1(c) disappears, and a crack-free film with pores of 200-500 nm is formed (Fig. 1(d)). Since the porous substrate is fully covered, the film thickness may be above the size of the pores (ca. 350 nm, Fig. 1(c)). However, the film cracks when the total dip-coating number is increased to m+n=3 (Figs. 1(e), (f)). There are more short cracks for sample TC1+2 than sample TC2+1, coinciding with the observation in Fig. 1(b) that the lateral shrinkage is more serious for PEG-containing gel film.
Figure 2 shows XRD patterns of the NiTi samples subjected to the same heat treatment (400 ℃ for 10 min and 500 ℃ for 10 min). For the polished and heat treated NiTi plate, apart from the strong peak of NiTi phase (JCPDS 18—899 or 19—850), a sharp diffraction peak of anatase (2θ=25.3°) and a small peak of rutile (27.5°) are detected (Fig. 2(a)). For the coating samples, with the increase of dip-coating layers, the peaks of anatase are enhanced, but the rutile peaks remain weak (Figs. 2(b), (c), (d)). This is because the sol-gel titania films annealed at around 500 ℃ are composed of anatase [20]. The weak shoulder at 2θ=43.3° is ascribed to Ni4Ti3 phase (JCPDS 39—1113) for the four heat treated samples [7,21]. Ni4Ti3 phase can be formed by precipitation at the grain boundaries of NiTi alloy during heat treatment [22]. Therefore, the heat treatment duration should be short (10 min) to avoid the detrimental effects of heat treatment on microstructure and properties of the NiTi samples.

Fig. 1 SEM images of polished and coated NiTi samples: (a) PC1+0; (b) PC0+1; (c) NaOH-HCl treated sample and its coating samples; (d) TC1+1; (e) TC2+1; (f) TC1+2
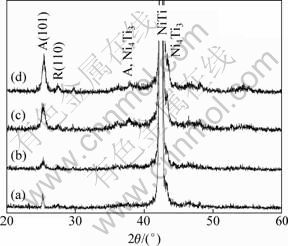
Fig. 2 XRD patterns of NiTi alloy samples heat treated at 400 ℃ for 10 min and at 500 ℃ for 10 min: (a) Polished and heat treated; (b) Sample TC1+1; (c) Sample TC1+2; (d) Sample TC2+2 (A: Anatase, R: Rutile)
Figure 3 shows XPS Ni 2p, Ti 2p and O 1s spectra of the coating sample TC1+1. The Ni 2p spectrum is flat, indicating that the titania coating completely covered NiTi substrate. Titanium on the surface presents mainly in the form of TiO2, with the binding energies of 458.5 eV (Ti 2p3/2) and 464.3 eV (Ti 2p1/2). The O 1s spectrum can be deconvoluted into three peaks. The main peak at 529.8 eV is attributed to TiO2, the small peak at 530.8 eV corresponds to Ti2O3, and the weak peak at 532.1 eV is related to hydroxyl groups[20]. The hydroxyl groups on the surface will increase hydrophilicity of the titania coated samples.
Hydrophilicity of the NiTi samples was evaluated by measuring their water contact angles (Fig. 4). The polished NiTi sample has a contact angle around 80°, and the sample TC1+1 has a much smaller contact angle about 20°. When subjected to UV illumination treatment for 15 min, the polished sample has a very small reduction in contact angle, but the sample TC1+1 becomes even more hydrophilic, with a contact angle of (9.2±3.2)°. Therefore, porous morphology and titania nature of the film and UV illumination treatment both contribute to the high hydrophilicity of sample TC1+1. The hydrophilic surface is beneficial for biological properties of biomaterials. For example, bioactivity and bone-bonding behavior of titanium can be improved [10,11], and platelet adhesion can be reduced, which in turn minimizes thrombogenic behavior [14].
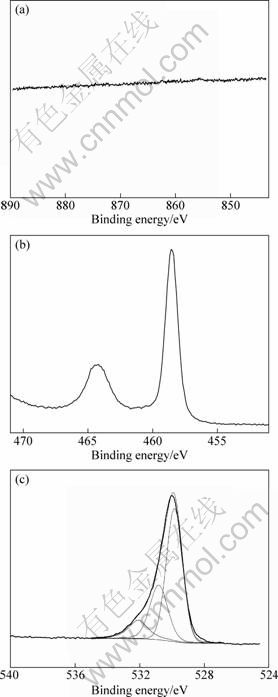
Fig. 3 XPS spectra of coating sample TC1+1: (a) Ni 2p; (b) Ti 2p; (c) O 1s
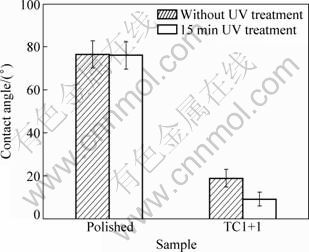
Fig. 4 Contact angles of polished NiTi sample and coating sample TC1+1 without and with 15 min UV irradiation treatment
Corrosion resistance of the NiTi samples was examined by the potentiodynamic polarization test in 0.9% NaCl solution. From the polarization curves (Fig. 5) it can be seen that the polished sample has a corrosion potential, φcorr=-0.15 V, and a pitting potential above 1.0 V, indicating good corrosion resistance. The coating sample TC1+1 has a lower φcorr (-0.25 V), but the pitting potential is still above 1.0 V. In addition, the passive current density of sample TC1+1 is about one order of magnitude smaller than that of the polished NiTi sample. Due to the dense inner layer, the good integrity and enough thickness, the sol-gel titania film of sample TC1+1 provides better protection for NiTi substrate in comparison with the natural oxide layer of the polished sample.
Titania coatings have been deposited onto titanium, NiTi and other alloys to improve their corrosion resistance and biocompatibility [2-9]. Among the vapor deposition techniques for TiO2 coatings, ion beam enhanced deposition can prepare films with high adhesion [3], but it is difficult for this line-of-sight process to form uniform coatings on the substrates with complex shapes. Sol-gel titania coatings have advantages of the independence of substrate shape, the high surface activity[3], and the good control of coating composition, thickness and topography [2,5].
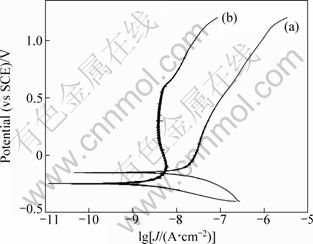
Fig. 5 Potentiodynamic polarization plots of polished NiTi sample (a) and coating sample TC1+1 (b)
Polyethylene glycol was used as a template to prepare porous sol-gel TiO2 films for biomedical, photocatalytic and other applications [8,9]. The addition of PEG increases viscosity of the titania sol, leading to the enlarged gel layer thickness and the increased tendency to crack of the film. The porous surface layer of NiTi alloy formed by the NaOH-HCl treatment can balance volume shrinkage of the gel film during the drying and heat treatment processes, thus improving integrity and adhesion of the sol-gel TiO2 film. The crack-free porous titania film of sample TC1+1 has improved hydrophilicity and corrosion resistance of the NiTi substrate.
4 Conclusions
1) Titania films with nano-sized pores are prepared on the NaOH-HCl pretreated NiTi alloy by sol-gel method. The titania films are composed of anatase, and a little Ni4Ti3 phase from the heat treated substrate is also detected.
2) For sample TC1+1, the titania film is crack-free and it completely covers the NiTi substrate. This sample is hydrophilic with a contact angle about 20°, and UV illumination treatment for 15 min further reduces the contact angle to (9.2±3.2)°.
3) In addition, this sample has a better corrosion resistance than the polished NiTi sample tested by the potentiodynamic polarization in 0.9% NaCl solution.
References
[1] YANG Da-zhi, WU Ming-xiong. Biomedical applications of Ni-Ti shape memory alloys [M]. Beijing: Metallurgy Industry Press, 2003. (in Chinese)
[2] LIU J X, YANG D Z, SHI F, CAI Y J. Sol-gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement [J]. Thin Solid Films, 2003, 429: 225-230.
[3] LIU Jing-tao, YANG Da-zhi, SHI Fei, CAI Ying-ji. Comparison of structure and properties of TiO2 films synthesized by sol-gel and ion beam on biomedical NiTi alloy [J]. Journal of Inorganic Materials, 2002, 17: 797-804. (in Chinese)
[4] CHENG F T, SHI P, MAN H C. A preliminary study of TiO2 deposition on NiTi by a hydrothermal method [J]. Surface & Coatings Technology, 2004, 187: 26-32.
[5] CHIU K Y, WONG M H, CHENG F T, MAN H C. Characterization and corrosion studies of titania-coated NiTi prepared by sol-gel technique and steam crystallization [J]. Applied Surface Science, 2007, 253: 6762-6768.
[6] KARPAGAVALLI R, ZHOU A H, CHELLAMUTHU P, NGUYEN K. Corrosion behavior and biocompatibility of nanostructured TiO2 film on Ti6Al4V [J]. Journal of Biomedical Materials Research, 2007, 83A: 1087-1095.
[7] FU T, LIU B G, ZHOU Y M, WU X M. Sol-gel titania coating on NiTi alloy with a porous titania film as interlayer [J]. Journal of Sol-Gel Science & Technology, 2011, 58: 307-311.
[8] BU S J, CUI C X, LIU X X, BAI L. Preparation of nanocrystalline porous titania films on titanium substrates by a sol-gel method with polyethylene glycol as a template [J]. Journal of Sol-Gel Science & Technology, 2007, 43: 151-159.
[9] ZHANG Jing-xian, ZHANG Xin-ping, SUN Xue-tong, LIU Ying-liang. Preparation of TiO2 coated biomedical porous NiTi alloys by sol-gel method [J]. Chinese Journal of Inorganic Chemistry, 2011, 27(2): 264-268. (in Chinese)
[10] IWASA F, HORI N, UENO T, MINAMIKAWA H, YAMADA M, OGAWA T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism [J]. Biomaterials, 2010, 31: 2717-2727.
[11] UENO T, YAMADA M, SUZUKI T, MINAMIKAWA H, SATO N, HORI N, TAKEUCHI K, HATTORI M, OGAWA T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model [J]. Biomaterials, 2010, 31: 1546-1557.
[12] MICHIARDI A, APARICIO C, RATNER B D, PLANELL J A, GIL J. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces [J]. Biomaterials, 2007, 28: 586-594.
[13] XU L C, SIEDLECKI C A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces [J]. Biomaterials, 2007, 28: 3273-3283.
[14] CHUN Y, LEVI D S, MOHANCHANDRA K P, CARMAN G P. Superhydrophilic surface treatment for thin film NiTi vascular applications [J]. Materials Science and Engineering C, 2009, 29: 2436-2441.
[15] BALAUR E, MACAK J M, TSUCHIYA H, SCHMUKI P. Wetting behaviour of layers of TiO2 nanotubes with different diameters [J]. Journal of Materials Chemistry, 2005, 15: 4488-4491.
[16] MIYAUCHI M, TOKUDOME H. Super-hydrophilic and transparent thin films of TiO2 nanotube arrays by a hydrothermal reaction [J]. Journal of Materials Chemistry, 2007, 17: 2095-2100.
[17] HOSONO E, MATSUDA H, HONMA I, ICHIHARA M, ZHOU H S. Synthesis of a perpendicular TiO2 nanosheet film with the superhydrophilic property without UV Irradiation [J]. Langmuir, 2007, 23: 7447-7450.
[18] FENG X J, ZHAI J, JIANG L. The fabrication and switchable superhydrophobicity of TiO2 nanorod films [J]. Angewandte Chemie International Edition, 2005, 44: 5115-5118.
[19] HAN Y G, WU G, WANG M, CHEN H Z. The growth of a c-axis highly oriented sandwiched TiO2 film with superhydrophilic properties without UV irradiation on SnO:F substrate [J]. Nanotechnology, 2009, 20: 235605.
[20] YU J G, ZHAO X J, DU J C, CHEN W M. Preparation, microstructure and photocatalytic activity of the porous TiO2 anatase coating by sol-gel processing [J]. Journal of Sol-Gel Science & Technology, 2000, 17: 163-171.
[21] KAYA M, ORHAN N, KURT B, KHAN T I. The effect of solution treatment under loading on the microstructure and phase transformation behavior of porous NiTi shape memory alloy fabricated by SHS [J]. Journal of Alloys & Compounds, 2009, 475: 378-382.
[22] NISHIDA M, WAYMAN C M, HONMA T. Precipitation processes in near-equiatomic Ti-Ni shape memory alloys [J]. Metallurgy Transaction A, 1986, 17: 1505-1515.
镍钛合金的溶胶-凝胶法多孔TiO2薄膜表面改性
付 涛1,2,吴晓明1,吴 锋3,罗 孟1,董兵辉1,纪 元1
1. 西安交通大学 生命科学与技术学院,生物医学信息工程教育部重点实验室,西安 710049;
2. 燕山大学 亚稳材料制备技术与科学国家重点实验室,秦皇岛 066004;
3. 西安交通大学 医学院,西安 710061
摘 要:在经过NaOH-HCl预处理的镍钛合金基体上,采用溶胶-凝胶法制备纳米多孔TiO2薄膜;当涂覆一层致密内膜和一层多孔外膜时,可得到无裂纹的薄膜(试样TC1+1)。X射线衍射表明,TiO2薄膜由锐钛矿组成,在热处理的基体中还检测到少量的Ni4Ti3相。X射线光电子谱分析表明,试样TC1+1的TiO2薄膜完全覆盖了镍钛合金基体。试样TC1+1的表面亲水,接触角约为20°,紫外光照处理15 min后接触角降低到(9.2±3.2)°。在0.9% NaCl溶液中的动电位极化实验表明,试样TC1+1的耐蚀性高于抛光的镍钛合金试样的。
关键词:镍钛合金;二氧化钛;溶胶-凝胶法;亲水性;耐蚀性
(Edited by LI Xiang-qun)
Foundation item: Project (xjj2011096) supported by the Fundamental Research Fund for the Central Universities, China; Project (201107) supported by the Open Project Program of State Key Laboratory of Metastable Materials Science and Technology, China; Project (50901058) supported by the National Natural Science Foundation of China
Corresponding author: FU Tao; Tel: +86-29-82669021; E-mail: taofu@mail.xjtu.edu.cn
DOI: 10.1016/S1003-6326(11)61370-8