低品位石煤矿中钒和碳的浮选回收
来源期刊:中国有色金属学报(英文版)2015年第11期
论文作者:王丽 孙伟 张庆鹏
文章页码:3767 - 3773
关键词:浮选;低品位;石煤;钒;碳
Key words:flotation; low grade; stone coal; vanadium; carbon
摘 要:在工艺矿物学的基础上,进行高碳含钒石煤浮选工艺研究。浮选工艺包括选碳流程和选钒流程,可以分别对碳和钒矿物进行富集。在碳浮选流程中,通过再磨工艺可以将碳和钒矿物有效分离。结果发现,浮选精矿中,钒的品位和回收率分别为1.32%和88.38%,尾矿产率为38.36%。碳精矿品位为30.08%,回收率为75.10%,可以直接作为燃料使用。对浮选产品进行酸法浸出实验,浮选产品的浸出率都在85%以上,说明该浮选工艺对浸出影响比较小。该浮选工艺可以减小钒浸出的处理量,降低冶炼成本,对石煤浮选工艺有借鉴作用。
Abstract: Flotation technology of high-carbon stone coal bearing vanadium was investigated based on mineralogical study. Carbon and vanadium flotation circuits were included in the flotation process for carbon and vanadium mineral concentrates. Carbon and vanadium minerals were efficiently separated via regrinding process in the carbon flotation circuit. The results show that the grade and recovery of V2O5 in flotation concentrate are 1.32% and 88.38%, respectively, and the tailings yield is 38.36%. Meanwhile, the grade and recovery of the carbon mineral are 30.08% and 75.10%, respectively, which may be utilized as the fossil fuels directly. The leaching rates of the flotation products are as high as 85%. The results demonstrate that there is no direct adverse effect of flotation process on vanadium leaching. This technology could potentially reduce cost and increase the treatment capacity of vanadium extraction and provide reference to stone coal flotation technology.
Trans. Nonferrous Met. Soc. China 25(2015) 3767-3773
Li WANG, Wei SUN, Qing-peng ZHANG
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
Received 1 December 2014; accepted 20 March 2015
Abstract: Flotation technology of high-carbon stone coal bearing vanadium was investigated based on mineralogical study. Carbon and vanadium flotation circuits were included in the flotation process for carbon and vanadium mineral concentrates. Carbon and vanadium minerals were efficiently separated via regrinding process in the carbon flotation circuit. The results show that the grade and recovery of V2O5 in flotation concentrate are 1.32% and 88.38%, respectively, and the tailings yield is 38.36%. Meanwhile, the grade and recovery of the carbon mineral are 30.08% and 75.10%, respectively, which may be utilized as the fossil fuels directly. The leaching rates of the flotation products are as high as 85%. The results demonstrate that there is no direct adverse effect of flotation process on vanadium leaching. This technology could potentially reduce cost and increase the treatment capacity of vanadium extraction and provide reference to stone coal flotation technology.
Key words: flotation; low grade; stone coal; vanadium; carbon
1 Introduction
As an important strategic metal, vanadium has been extensively applied in the fields of steel and alloy materials industries due to their excellent high tensile strength, hardness and fatigue resistance [1,2]. Approximately 80% of the world’s vanadium is used in metallurgical purpose in alloy steels, and the other 20% is used in chemical purpose [3]. Vanadium occurs naturally in over 50 different minerals and in fossil fuel deposits rather than in its pure state [3]. Vanadium resources in China primarily exist in vanadium–titanium magnetite ore and stone coal [4]. The gross reserve of V2O5 in stone coal is 118 million tons, accounting for higher than 87% of the vanadium reserve in China and exceeding the total vanadium reserve in other countries [5,6]. Therefore, stone coal has aroused considerable attention as an important vanadium-bearing resource.
Currently, vanadium extraction technology from stone coal mainly includes roasting, leaching, ion purification, precipitation and calcinations [2,7]. Due to the low vanadium grade in stone coal (0.13% to 1.2%), traditional vanadium extraction processes face the problems of large ore tonnage, high energy consumption and acid consumption [8]. The market price of V2O5 decreased to approximately 75000 CNY per ton in 2013, which is close to its production cost. As the vanadium market price decreases, many vanadium enterprises in China must reduce or cease production. In recent years, a large amount of work has been conducted to improve vanadium recovery and reduce its production cost. Numerous studies have been reported on leaching technologies, such as direct acid, recycling alkali and calcified roasting-carbonate leaching [9]. Meanwhile, some efforts have been emphasized on vanadium beneficiation before being further subjected to melting processing.
If the V2O5 grade of stone coal increases by 0.1%, the production cost of 1 t of V2O5 would decrease by approximately 4000 CNY, increasing profits by 29% [8]. As a result, only relatively high-grade ore (>0.5% V2O5) has economic value and is worth smelting, which only accounts for 40% of the total ore [10]. Most of the mined stone coals are of low grade and require beneficiation to obtain vanadium concentrate. If vanadium beneficiation is successful, a reasonable amount of production cost will be reduced. ZHAO et al [8] attempted to pre-concentrate vanadium value from stone coal by gravity separation with a shaking table after decarbonization, achieving good results. During the decarbonization stage, the carbon thermal energy in the stone coal can be utilized, and the V2O5 grade increased to some extent. However, the combustibility of stone coal was very poor due to its low carbon content (13.44%) and cannot be directly used as fuel to generate heat in practice. Much information on stone coal beneficiation is available in the literature. XIANG et al [11] investigated the enrichment of vanadium value in stone coal from the Xinjiang Province in China. The results demonstrated that the primary minerals bearing vanadium were muscovite and tourmaline, while the main gangue mineral was quartz. Vanadium concentrate was eventually obtained with a V2O5 grade of 3.20% and a recovery of 74.50% using a combination of wet sieving and flotation.
The objective of this work was to pre-concentrate carbon and vanadium from high carbon stone coal obtained from the Shanxi Province, China. Mineralogical studies were performed to confirm the main occurrence of vanadium and carbon and to identify the liberation of these minerals. The designed process flowsheet is composed of a carbon circuit and a vanadium circuit. Flotation condition tests were then conducted to study the effects of particle size, pulp pH and reagent dosage on the recovery and grade of carbon and vanadium.
2 Experimental
2.1 Materials and reagents
The experimental sample of stone coal was obtained from the Shanyang region of Shanxi Province in China. Ore samples were crushed by a laboratory jaw crusher, followed by a roll crusher to less than 3 mm in size. The crushed product was then ground in a ball mill to a pulp density of 70%. The ground product was screened through a 420 μm sieve to remove oversized material. Mixed oil (MO) and mixed amines (MA) were used as a carbon collector and a vanadium collector, respectively. Sodium silicate (SS) was used as a dispersant, terpenic oil (TO) was used as a frother, and H2SO4 was used for pH adjustment.
2.2 Characterization methods
Factors such as mineralogy and particle size distribution are important for choosing suitable concentration methods for stone coal beneficiation. Chemical composition was characterized by X-ray fluorescence spectrometry (XRF) technique using a Philips spectrometer. Mineral phase composition was investigated by powder X-ray diffraction (XRD, D8-ADVANCE, Bruker Co., Germany). Vanadium phase analysis was carried out according to the sequential extraction procedures. Optical microscopy (LEICA DMLB, Leica, Wetzlar, Germany) and scanning electron microscope with energy dispersive spectrometer (SEM–EDS, JSM-6490LV, JEOL Co. Japan) were used to characterize microstructure of the stone coal. Bulk chemical analysis of the ore was conducted to examine the distribution of vanadium and carbon in each size fraction. The sample was classified into different size fractions i.e., <38, 38-50, 50-74, 74-154, 154-600, 600-1500 and >1500 μm using standard laboratory sieves.
2.3 Experimental procedure
Flotation was conducted in a Denver laboratory sub-aeration cell. Rougher flotation was performed using a 1.5 L-capacity cell, whereas cleaner flotation was conducted with a 1 L-capacity cell. The flotation process flowsheet of stone coal is shown in Fig. 1. Carbon flotation was performed at 30% pulp density and 1400 r/min at pH 7. Carbon was preferentially floated in virtue of its natural floatability, and then a cleaner flotation was conducted to obtain high carbon recovery. After carbon floatation, vanadium minerals were concentrated using a two-stage flotation process. The flotation products were filtered, thermally dried, weighed and analyzed by XRF.

Fig. 1 Process flow-sheet stone coal
3 Results and discussion
3.1 Mineralogical characteristics
3.1.1 Chemical composition analysis
XRF chemical analysis of stone coal indicates that samples contain 0.92% V2O5, 4.03% Al2O3, 2.87% Fe2O3, 2.62% K2O, 69.44% SiO2, 5.42% SO3 and 14.12% C. XRD pattern of the stone coal sample is shown in Fig. 2. According to XRD analysis, the mineral phases presented in the ore samples are quartz, muscovite, pyrite, feldspar and montmorillonite.
3.1.2 Occurrence of vanadium
Muscovite is the primary carrier of vanadium in stone coal. Vanadium generally exists as trivalent vanadium (V(III)), which substitutes for Al3+ in the dioctahedron of mica group minerals as an isomorphism, as they possess a similar ionic radius and electronegativity and the same coordination number [1]. Furthermore, vanadium exists in an adsorption form on iron oxide, titanium oxide, tourmaline and garnet [12]. Mineralogical results of the stone coal samples indicate that vanadium mainly exists in muscovite (0.12%), garnet (0.02%), iron-titanium oxide and clay minerals (0.26%). Stone coal microstructure and phase structure were analyzed by SEM-EDS (Fig. 3 and Table 1). The desired minerals containing muscovite and iron-titanium oxides bearing vanadium both appeared to be associated with quartz. Figure 3(a) suggests that the shape of these particles is irregular. Many microscale granular muscovite minerals bearing vanadium (V-muscovite) spread over quartz in greater detail. Iron-titanium oxides bearing vanadium (V-iron, Fig. 3(b)) are also identified but not determined by XRD. V-iron minerals exhibit an irregular shape and closely coexist with quartz. Most of these minerals cluster together, while others scatter among quartz.
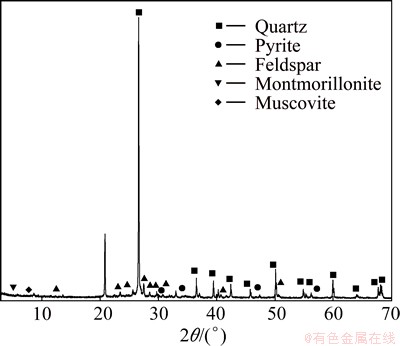
Fig. 2 XRD pattern of stone coal
3.1.3 Occurrence of carbon
Due to low-grade carbon (high ash and low calorific value), stone coal is known as inferior coal [13]. In China, most of the stone coal should be pre-concentrated before being made available for combustion. Mineralogical results indicate that carbon is in the form of carbonate (1.56%) and amorphous carbon (98.44%). Optical images of stone coal are shown in Fig. 4, clearly suggesting that the carbon does not exist as individual particles. Some of the carbon minerals (Fig. 4(a)) in the form of needle-like structures are closely associated with quartz, as they possess fine dissemination sizes (<5 μm). Some of the other carbon minerals (Fig. 4(b)) are irregular in shape and size and are associated with quartz with coarse dissemination ore sizes (5-50 μm).
3.1.4 Chemical composition analysis
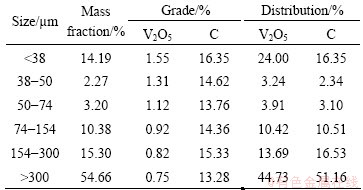
The distribution of carbon and vanadium in ore is given in Table 2. The particle size distribution of the stone coal is uneven, and the yield of coarse particles with sizes greater than 300 μm accounts for nearly 55%. Vanadium and carbon minerals occur in all fraction sizes. Moreover, the grade of V2O5 decreases with increasing size fraction, indicating that V2O5 mainly exists in fine clay particles, while carbon minerals are evenly distributed across the ore. From the SEM and optical results, the ore should be milled before flotation to further liberate the valuable, single mineral.
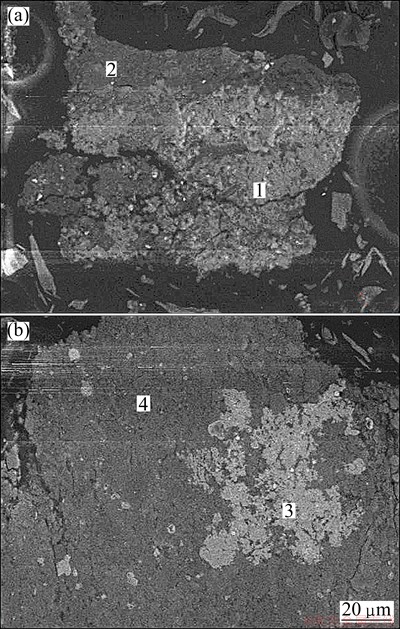
Fig. 3 SEM images of ore sample stone coal
Table 1 EDS analysis result of ore sample stone coal in Fig. 3 (mass fraction, %)
3.2 Effect of particle size
Particle size distribution is an important parameter in flotation [14]. A suitable fine grinding can liberate a valuable, single mineral from gangue and eliminate fine grinding to avoid slime formation. Carbon and vanadium minerals are not liberated from the gangue minerals based on our mineralogical study. Crushed samples were ground with a ball mill (ADVAN-TEC, No. 5C). The rotation speed was maintained at 400 r/min, and the grinding time period ranged from 2 to 18 min. The effect of the grinding time on the fineness is presented in Fig. 5. The results show that the yield of the <74 μm size fraction increases from 23.43% to 100% as the grinding time increases from 2 to 18 min.

Fig. 4 Optical micrographs of ore samples
Table 2 Vanadium and carbon distribution in ore sample
The rougher flotation of carbon was performed using a pulp pH of 7.5 and with 2000 g/t SS, 1000 g/t MO and 80 g/t TO, and cleaner flotation was conducted with 500 g/t MO and 60 g/t TO. The effects of a rougher pulp slurry particle size distribution on carbon recovery and grade in the carbon circuit are shown in Fig. 6. The C grade in the C1 product decreases sharply and increases slowly in the Cc product with increasing grinding fineness. The recovery of C decreases when the ratio of slurry particles <74 μm is above 38.89%. Thus, the feed particle size distribution of 38.89% of <74 μm grinding fineness selected to perform subsequent flotation tests.
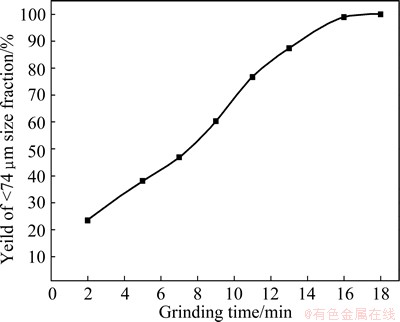
Fig. 5 Effect of grinding time on grinding fineness
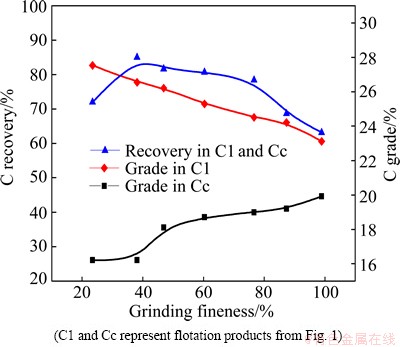
Fig. 6 C recoveries and grades as function of grinding fineness
3.3 Effect of regrinding
Regrinding in flotation circuits allows the overall energy consumption to be reduced by operating the primary grinding stage at relatively coarse particle sizes while re-grinding the rougher concentrate. Other streams, such as cleaner–scavenger concentrates or cleaner tails are typically re-circulated to the regrinding stage to achieve the particle size required for the final upgrading in the cleaning stage [15]. Middling products often contain higher proportions of composites, and as a result, increased regrinding may be necessary [16].
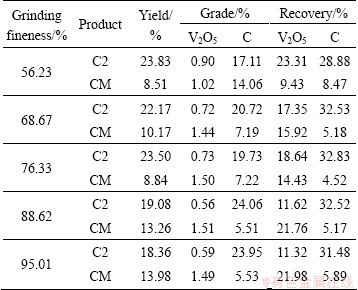
The particle size of Cc (Fig. 1) is relatively coarse, resulting in poor concentration in the laboratory. Hence, Cc regrinding was conducted. Further concentration was performed and the results are shown in Table 3. Carbon and vanadium minerals are efficiently separated in the regrinding process. With the increase of grinding fineness, the grade and recovery of C increase, while the grade and recovery of V2O5 decrease in the C2 product. When the grinding fineness is greater than 88.62% of <74 μm, the decrease in the C and V2O5 grades becomes smooth. As a result, 88.62% of <74 μm was adopted in regrinding.
Table 3 Products of analysis of C2 concentrate
3.4 Particle size distribution of decarburized sample
Figure 7 shows the V2O5 grade in each size fraction of the decarburized samples compared with that of raw ore. Through the decarburization process, the V2O5 grade of the fine fraction (<38 μm) significantly increased, while that of the coarse fraction (>74 μm) decreased to a degree. Meanwhile, the maximum difference in the V2O5 grade among each size fraction increases from 0.75% to 2.14%. Thus, from Fig. 7, appropriate conditions for fine particle minerals bearing vanadium must be chosen to conduct vanadium flotation.
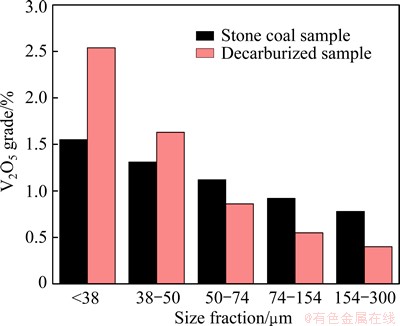
Fig. 7 V2O5 grade in each raw ore and decarburized sample size fraction
3.5 Effect of rougher pH and MA dosages on vanadium flotation
Based on the preferential flotation of carbon, the influence of pulp pH on the recovery and grade of V2O5 in vanadium concentrate is shown in Fig. 8(a). These data indicate that with increasing pH, the V2O5 grade decreases sharply, while V2O5 recovery increases slightly. Therefore, the recommended rougher pH value is 6, resulting in a 3.32% V2O5 grade and an 18.32% V2O5 recovery from vanadium concentrate.
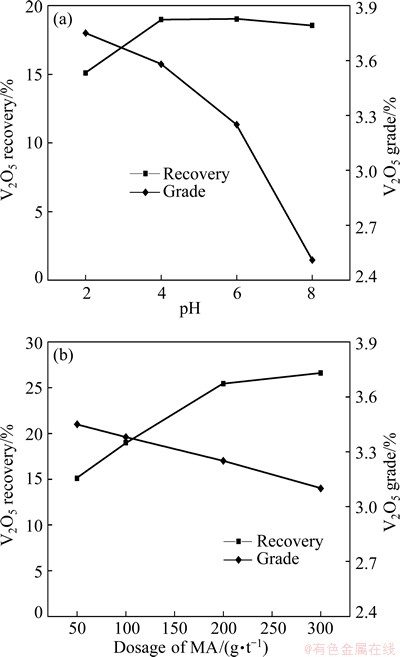
Fig. 8 V2O5 recovery and grade in V rougher concentrate as function of pH (a) and MA dosage (b)
With a pulp pH of 6, the effect of MA dosage on V2O5 recovery and grade in the vanadium concentrate is shown in Fig. 8(b). A rapid increase in V2O5 recovery is obtained when the initial MA dosage is increased from 50 to 200 g/t, whereas only a slight increase in V2O5 recovery is observed when the initial MA dosage exceeds 200 g/t. As a result, 200 g/t MA was determined during the following rougher operation, in which a concentrate with 25.43% V2O5 recovery and 3.25% V2O5 grade was obtained.
3.6 Flotation process and leaching result
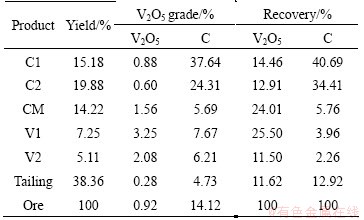
The process flowsheet used herein is presented in Fig. 1, and the results are listed in Table 4. The V2O5 grade and recovery in the flotation concentrate are 1.32% and 88.38%, respectively. The V2O5 grade in tailing is only 0.28%, which is much lower than that of the ore, whereas the rejection rate is up to 38.36%. Furthermore, using a two-stage grinding process, a comprehensive carbon concentrate (including C1 and C2) can be obtained with a 75.10% C recovery and a 30.08% grade. The carbon concentrations should probably be leached separately from the VC and their leaching residue burned as fuel due to their much higher calorific values (C1 9723 J/g, C2 7513.8 J/g) when compared to feed ore (3389.4 J/g). Therefore, the flotation process described herein not only improves V2O5 grade but also saves fuel for the subsequent leaching process.
Table 4 Products analysis of concentrate and tailing
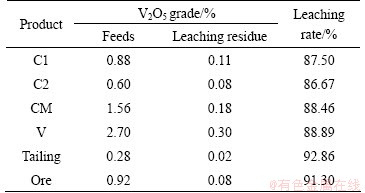
To investigate the effects of flotation reagents and other factors on follow-up metallurgy processes, the vanadium leaching rates from the flotation products were investigated using a direct acid leaching process, with 175 g/t H2SO4, 3 kg/t NaCl, 16 g/t CaF for C1, C2 and CM and 30 g/t CaF for V, a leaching temperature of 95 °C and a leaching time of 8 h. The flotation product leaching rates are listed in Table 5. The results show leaching rates of up to 85%, indicating no adverse flotation process effects on vanadium leaching, most likely due to lower reagent dosages when compared to those used in other vanadium leaching technology.
Table 5 Leaching efficiencies of flotation products
4 Conclusions
1) An investigation was conducted to develop an approach to pre-concentrate vanadium from stone coal. Carbon is primarily floated off before vanadium mineral flotation. A systematic evaluation of flotation parameters on C and V2O5 grades and recoveries was conducted, including grinding fineness, pulp pH and reagent dosage.
2) 38.36% of the feed ore was rejected, and the loss of V2O5 was only 11.62% after this flotation process. The pre-concentration process reduces follow-up smelting costs in terms of thermal energy and leaching reagent dosage. Moreover, the leaching carbon concentrate residue can be used as fuel. The proposed flotation process demonstrated no adverse effects on vanadium leaching.
References
[1] ZHANG Yi-min, HU Yang-jia, BAO Shen-xu. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine [J]. Minerals Engineering, 2012, 30: 95-98.
[2] HE Dong-sheng, FENG Qi-ming, ZHANG Guo-fan, OU Le-ming, LU Yi-ping. An environmentally-friendly technology of vanadium extraction from stone coal [J]. Minerals Engineering, 2007, 20(12): 1184-1186.
[3] MOSKALYK, R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793-805.
[4] ZHU Xiao-bo, ZHANG Yi-min, HUANG Jing, LIU Tao, WANG Yi. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal [J]. International Journal of Mineral Processing, 2012, 114-117: 1-6.
[5] YE Pu-hong, WANG Xue-wen, WANG Ming-yu, FAN Ye-ye, XIANG Xiao-yan. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching [J]. Hydrometallurgy, 2012, 117-118: 108-115.
[6] WANG Li, SUN Wei, LIU Rui-qing, GU Xiao-chuan. Flotation recovery of vanadium from low-grade stone coal [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1145-1151.
[7] ZHENG Li, LI Qing-gang, XIAO Lian-sheng. Extraction of vanadium from the leach solution of stone coal using ion exchange resin [J]. Hydrometallurgy, 2009, 97(3): 194-197.
[8] ZHAO Yun-liang, ZHANG Yi-min, LIU Tao, CHEN Tie-jun, BIAN Ying, BAO Shen-xu. Pre-concentration of vanadium from stone coal by gravity separation [J]. International Journal of Mineral Processing, 2013, 121: 1-5.
[9] ZENG Xi, WANG Fang, ZHANG Hui-feng, CUI Li-jie, YU Jian, XU Guang-wen. Extraction of vanadium from stone coal by roasting in a fluidized bed reactor [J]. Fuel, 2014, 142: 180-188.
[10] DENG Zhi-gan, WEI Chang, FAN Gang, LI Min-ting, LI Cun-xiong, LI Xing-bin. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s118-s122.
[11] XIANG Pi, FENG Qi-ming, NIU Yin-jian, PAN An-xin. Enrichment of vanadium from stone coal in Aksu vanadium mine by ore dressing method [J]. Materials Research and Application, 2010, 1: 65-70. (in Chinese)
[12] LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Min-ting, LI Xing-bin, FAN Gang. Recovery of vanadium from black shale [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s127-s131.
[13] ZHANG Yin-min, BAO Shen-xu, LIU Tao, CHEN Tie-jun, HUANG Jing. The technology of extracting vanadium from stone coal in China: History, current status and future prospects [J]. Hydrometallurgy, 2011, 109(1): 116-124.
[14] ZHANG Hai-jun, LIU Jiong-tian, CAO Yi-jun, WANG Yong-tian. Effects of particle size on lignite reverse flotation kinetics in the presence of sodium chloride [J]. Powder Technology, 2013, 246: 658-663.
[15] YIANATOS, J, BERGH L, VINNETT L,  F. Modeling of residence time distribution in regrinding Vertimill [J]. Minerals Engineering, 2013, 53: 174-180.
F. Modeling of residence time distribution in regrinding Vertimill [J]. Minerals Engineering, 2013, 53: 174-180.
[16] QI G W, PARENTICH A, LITTLE L H, WARREN L J. A QEM*SEM study of the flotation of composite particles [J]. International Journal of Mineral Processing, 1992, 34(1): 71-82.
王 丽,孙 伟,张庆鹏
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:在工艺矿物学的基础上,进行高碳含钒石煤浮选工艺研究。浮选工艺包括选碳流程和选钒流程,可以分别对碳和钒矿物进行富集。在碳浮选流程中,通过再磨工艺可以将碳和钒矿物有效分离。结果发现,浮选精矿中,钒的品位和回收率分别为1.32%和88.38%,尾矿产率为38.36%。碳精矿品位为30.08%,回收率为75.10%,可以直接作为燃料使用。对浮选产品进行酸法浸出实验,浮选产品的浸出率都在85%以上,说明该浮选工艺对浸出影响比较小。该浮选工艺可以减小钒浸出的处理量,降低冶炼成本,对石煤浮选工艺有借鉴作用。
关键词:浮选;低品位;石煤;钒;碳
(Edited by Yun-bin HE)
Foundation item: Project (2012BAB07B05) supported by the National Science & Technology Support Program during “Twelfth Five-Year” Plan Period
Corresponding author: Wei SUN; Tel: +86-731-88830482; E-mail: sunmemghu@csu.edu.cn
DOI: 10.1016/S1003-6326(15)64020-1

