钼酸铵溶液镁盐沉淀法除砷的热力学分析
杨亮,赵中伟,何利华,霍广生,陈爱良
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要:湿法处理镍钼矿所得钼酸铵溶液砷含量较高。针对镁盐沉淀法除砷的工艺,进行热力学分析。根据同时平衡原理和质量守恒定律,进而绘制在25 ℃ 时Mg-NH4-As-H2O系热力学平衡图,并考察工艺参数对除砷的影响。研究结果表明:溶液中总氨浓度的升高及镁盐用量增大,平衡时砷含量降低。当钼酸铵溶液中 [N]T=5 mol/L,[As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,整个pH范围存在4个平衡固相的稳定区即MgHAsO4稳定区(4.0<pH<6.0),MgNH4AsO4稳定区(6.0<pH<9.5),MgNH4AsO4和Mg(OH)2稳定区(9.5<pH<11.7)及Mg(OH)2稳定区(pH>11.7);当溶液pH为9时,砷质量浓度可除至2×10-6 g/L,溶液中残留镁浓度为0.02 mol/L。
关键词:镍钼矿;除砷;砷酸铵镁;热力学
中图分类号:TF841.2 文献标志码:A 文章编号:1672-7207(2012)05-1610-06
Thermodynamics analysis on removal of arsenic from ammonium molybdate solution by chemical precipitation with magnesium salt
YANG Liang, ZHAO Zhong-wei, HE Li-hua, HUO Guang-sheng, CHEN Ai-liang
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The coarse ammonium molybdate solution obtained by hydrometallurgical treatment of Ni-Mo ore contains high arsenic. Thermodynamic analysis of removing arsenic from the ammonium molybdate solution with addition of MgCl2 was studied. According to the principle of simultaneous equilibrium and the law of conservation of mass, thermodynamic equilibrium diagrams of Mg-NH4-As-H2O system were established at 25 ℃. The effect of technical conditions on removal of arsenic was investigated. The results indicate that the concentration of arsenic decreases with the increase of total ammonium concentration and dosage of MgCl2. There are four stable areas of various solid phases in the whole pH of the system, when total ammonium is 5 mol/L, total arsenic is 0.1mol/L and total Mg is 0.12 mol/L in the system. The first one is MgHAsO4 (4.0<pH<6.0), the second one is MgNH4AsO4 (6.0<pH<9.5), the third one is MgNH4AsO4 and Mg(OH)2 (9.5<pH<11.7), and the forth one is Mg(OH)2 (pH>11.7). Adjusting the pH to 9, the arsenic concentration can be thermodynamically decreased to 2×10-6 g/L and residual magnesium concentration is 0.02 mol/L in the solution.
Key words: Ni-Mo ore; arsenic removal; MgNH4AsO4; thermodynamics
在我国贵州遵义和湖南张家界,储存有大量的镍钼矿资源[1-3]。镍钼矿是一种新型的钼矿资源,镍钼矿中钼含量高达约为4%,砷含量约为0.8%[4-5]。浸出镍钼矿时[6],砷与钼同时进入浸出液中。Zhao等[7]开发了N-235萃取提钼工艺,由于砷与钼形成杂多酸离子,与钼共萃取而进入钼酸铵溶液中。典型溶液含钼的质量浓度约为90 g/L时,砷的质量浓度约为8 g/L,砷钼质量比高达0.09。但是钼冶金产品中,砷是严格控制的杂质元素,其中钼酸铵产品GB 3460—2007中,MSA-0级钼酸铵产品中规定砷质量浓度小于5×10-6 g/L,因此,需要采用适当的方法深度除砷。从钼酸铵溶液中除砷的方法主要有离子交换法[8]、溶剂萃取 法[9]、吸附法[10-11]、沉淀法等。离子交换法难以处理砷含量较高以及成分复杂的溶液。采用伯铵和TBP萃取除砷则存在砷萃取率不高且钼损较大的缺点。采用铁盐吸附法除砷,钼损失较大且吸附剂的再生及回收较为困难。铵镁盐沉淀法具有除砷效果好、钼损失率低、滤渣过滤性能好及操作简单等优点而被广泛采用。但是对除砷过程的平衡问题特别是理论研究不太透彻,仅凭经验判断来控制工艺条件。在此,本文作者通过热力学分析,绘制出镁盐除砷过程的热力学平衡图并进行热力学分析,以期能深化认识以及为生产实践提供一定的理论指导。
1 热力学数据处理及计算
钼酸铵溶液中加入氯化镁溶液,砷形成溶解度小的砷酸盐而除去。考虑到钼酸铵溶液为氨性体系,分析了氨浓度较高时([N]T=1~5 mol/L)除砷的热力学过程。考察了不同总氨浓度及不同镁盐加入量对溶液离子组成与固相平衡的关系。
在NH4+-Mg2+-AsO43--H-H2O体系中,除了生成MgNH4AsO4外,还可能存在固相MgHAsO4(s),Mg3(AsO4)2(s)和Mg(OH)2(s)。由于缺乏MgNH4AsO4的热力学参数,采用化学中广泛应用的同系线形规律进行估算[12]。估算时所采用的有关化合物的热力学数据[13]如表1所示。
利用表1中的热力学数据,将磷酸盐的?GΘ与相应的砷酸盐的?GΘ作图,可以看出数据基本上落在1条直线上。回归线性方程为: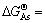
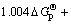
 ,其线性相关系数R2=0.999 9。其中:
,其线性相关系数R2=0.999 9。其中: 为砷酸盐的吉布斯自由能;
为砷酸盐的吉布斯自由能;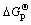 为磷酸盐的吉布斯自由能。由文献[14]可知反应Mg2++NH4++PO43-+6H2O= MgNH4PO4·6H2O,反应平衡常数K=1013.15,可算出MgNH4PO4·6H2O的?GΘ为-3 038.24 kJ/mol,代入回归方程可算出MgNH4AsO4·6H2O的?GΘ为-2 668.74 kJ/mol. 进而求得反应Mg2++NH4++AsO43-+6H2O= MgNH4AsO4·6H2O 的生成吉布斯自由能?GΘ= -59.81 kJ/mol。由关系式?GΘ = -RTln K,算出MgNH4AsO4·6H2O 的溶度积常数Ksp=10-10.48。
为磷酸盐的吉布斯自由能。由文献[14]可知反应Mg2++NH4++PO43-+6H2O= MgNH4PO4·6H2O,反应平衡常数K=1013.15,可算出MgNH4PO4·6H2O的?GΘ为-3 038.24 kJ/mol,代入回归方程可算出MgNH4AsO4·6H2O的?GΘ为-2 668.74 kJ/mol. 进而求得反应Mg2++NH4++AsO43-+6H2O= MgNH4AsO4·6H2O 的生成吉布斯自由能?GΘ= -59.81 kJ/mol。由关系式?GΘ = -RTln K,算出MgNH4AsO4·6H2O 的溶度积常数Ksp=10-10.48。
除砷过程中发生的化学反应及表观化学平衡常数如表2所示。
表1 磷和砷化合物的热力学数据
Table 1 Thermodynamic data of compounds containing P and As
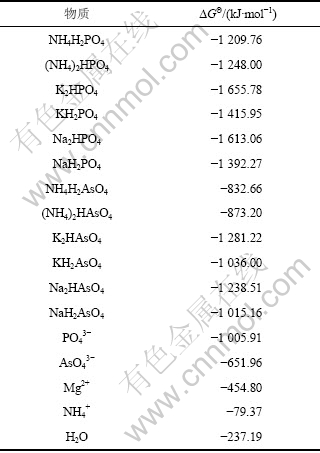
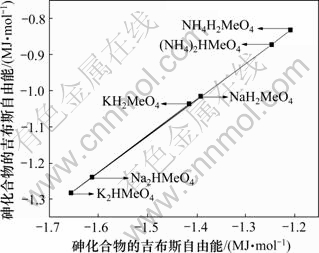
图1 砷盐与磷盐?GΘ间的同系线性关系
Fig.1 Linearity relation of ?GΘ between arsenic compounds and phosphorus compounds
由于该体系成分较为复杂,且缺乏相关物质的活度系数,故本计算均以物质的浓度代替活度。由上述平衡关系式可以看出溶液中的N,Mg和As的形态有H2AsO4-,HAsO42-,AsO43-,MgH2AsO4+,MgHAsO4,MgAsO4-,Mg2+,NH3,NH4+和MgOH+。由表2可知:
各离子平衡浓度应满足下列方程:
[H3AsO4]=[H +]×[H2AsO4-]×102.2 (1)
[H2AsO4-]=[H+]×[HAsO42-]×106.98 (2)
HAsO42-]=[ H+]×[AsO43-]×1011.5 (3)
[MgH2AsO4+]=[ Mg2+]×[H2AsO4-]×101.52 (4)
[MgHAsO4]=[ Mg2+]×[HAsO4-]×102.86 (5)
[MgAsO4-]=[ Mg2+]×[AsO43-]×106.34 (6)
[NH4+]=[ NH3]×[H+]×109.24 (7)
[MgOH+]=[ Mg2+]×[OH-]×102.58 (8)
表2 除砷时的化学反应及其在25 ℃下的平衡常数
Table 2 Equilibrium constant and chemical reaction in process of arsenic removal (25 ℃)

随着溶液pH的变化,溶液中会出现不同的固相组成,因此它们的溶解平衡需要分别加以考虑。当体系处于MgHAsO4稳定区时,则有平衡关系式:
[Mg2+]×[HAsO42-]=10-5.66 (9)
溶液中各离子浓度满足式(1)~(9),当体系处于MgNH4AsO4稳定区时,则有平衡关系式:
[Mg2+]×[NH4+]×[AsO43-10-10.48 (10)
溶液中各离子浓度则满足式(1)~(8)和式(10),当体系处于Mg3(AsO4)2稳定区时,则有平衡关系式:
[Mg2+]3×[AsO43-]2=10-22.32 (11)
溶液中各离子浓度应满足式(1)~(8)和式(11),当体系处于Mg(OH)2 稳定区时,则有平衡关系式:
[Mg2+]×[OH-]2=10-11.15 (12)
溶液中各离子浓度则满足式(1)~(8)和式(12)。
根据质量平衡原理可知:溶液中总氮、总砷和总镁浓度分别为:
[Mg]T=[Mg2+]+[ MgH2AsO4+]+[ MgHAsO4]+
[ MgAsO4-]+[ MgOH+]
[N]T=[NH4]+[NH3]
[As]T=[H3AsO4]+[ H2AsO4-]+[ HAsO42-]+[ AsO43-]+
[ MgH2AsO4+]+[ MgHAsO4]+[ MgAsO4-]
因此,根据同时平衡原理,在固定总氨[N]T、总砷[As]T以及总镁[Mg]T的条件下,可计算出各固相成分稳定存在的pH区间,以及溶液中各离子的平衡 浓度。
2 结果与讨论
2.1 溶液中总氨浓度[N]T对除砷的影响
图2所示为[As]T=0.1 mol/L,[Mg]T=0.1 mol/L时,不同总氨浓度时lg[As]T与pH关系。从图2可知:当溶液中总氨浓度为[N]T=1 mol/L,[Mg]T=[As]T=0.1 mol/L时,随着体系pH的逐渐增大,溶液中稳定存在的固相依次为MgHAsO4,MgNH4AsO4,Mg3(AsO4)2和Mg(OH)2,Mg(OH)2,其中MgNH4AsO4沉淀稳定区域为6.7<pH<10.5。固定其他条件不变,增大总氨浓度至[N]T=5 mol/L时,随pH的变化,溶液中稳定存在的固相依次为MgHAsO4,MgNH4AsO4,MgNH4AsO4和Mg(OH)2,Mg(OH)2,其中MgNH4AsO4沉淀稳定区域为6.0<pH<10.7,MgNH4AsO4和Mg(OH)2共沉淀的稳定区间为10.7<pH<11.8。通过对比可以发现:随着体系总氨浓度的提高,MgNH4AsO4沉淀的稳定区域变大,且Mg3(AsO4)2和Mg(OH)2共沉淀区域消失,取而代之的是MgNH4AsO4和Mg(OH)2共沉淀。从图2还可以发现:控制溶液的pH使体系处于MgNH4AsO4沉淀的稳定区时,除砷效果最好,而且溶液中总氨浓度越高,则平衡时溶液中总砷浓度越低。处理镍钼矿得到的钼酸铵溶液总氨浓度较高(约为5 mol/L),与钼酸钠溶液中除砷不同,无需调整氨浓度便可达到较好的除砷效果。
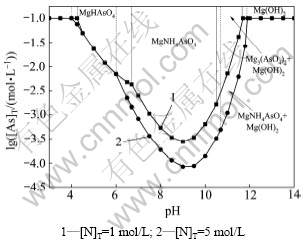
图2 [As]T=0.1 mol/L、[Mg]T=0.1 mol/L时,不同总氨浓度时lg[As]T与pH关系
Fig.2 lg[As]T-pH at different total ammonium concentrations ([As]T=0.1 mol/L, [Mg]T=0.1 mol/L)
2.2 镁盐用量对除砷的影响
处理镍钼矿得到的钼酸铵溶液中总氨浓度约为5 mol/L,砷约为0.1 mol/L。在实际操作中,加入理论用量的MgCl2往往难以将砷除尽,因此,实践中往往加入过量的氯化镁。参考生产实际参数,考察镁加入量为理论量1.2倍时,平衡时体系各组分随溶液pH的变化关系。
图3所示为[N] T=5 mol/L, [As]T=0.1 mol/L时,不同镁盐用量时lg(As)T与pH关系。从图3可知:控制适当的pH使得体系处于MgNH4AsO4稳定区域时,随着镁盐加入量的增大,平衡时溶液砷含量更低,除砷效果更好。随着镁盐加入量的增大,MgNH4AsO4沉淀的稳定区域缩小,其稳定区域由原来的6.0<pH<10.7缩小至6.0<pH<9.5,在图3中显示即由①线向左平移至②线位置,而MgNH4AsO4与Mg(OH)2共沉淀稳定区增大。因此,钼酸铵溶液中加入过量的氯化镁除砷时,需要严格控制溶液的pH。倘若钼酸铵溶液pH大于9.5时,将形成MgNH4AsO4与Mg(OH)2共沉淀,不仅溶液中砷含量增大,除砷效果变差,而且由于生成的Mg(OH)2沉淀比较黏稠,容易吸附溶液中的钼而造成钼的回收率下降。从图3还可以发现:当加入1.2倍理论量的氯化镁除砷时,控制溶液pH为9时,除砷效果较好,热力学上可将砷质量浓度降低至2×10-6 g/L左右,此时,由图5和图6可知:溶液中残留的砷主要以MgAsO4-离子形态存在,残留的镁则主要以游离的Mg2+存在,且由图4可知:残留的镁浓度约为0.02 mol/L(即0.48 g/L)。当溶液pH位于9.5和11.7之间,平衡固相组成为MgNH4AsO4和Mg(OH)2,且随着pH增大,共沉淀中砷酸铵镁逐渐减少,氢氧化镁含量逐渐增大(见图7)。当溶液pH大于11.7时,溶液中稳定存在的是氢氧化镁沉淀。实际上,处理镍钼矿所得的钼酸铵溶液pH约为9,无需调整溶液的pH即可达到较好的除砷效果。

图3 [N] T=5 mol/L, [As]T=0.1 mol/L时,不同镁盐用量时lg(As)T与pH关系
Fig.3 lg[As]T-pH at different dosages of magnesium salt ([N]T=0.1 mol/L, [As]T=0.1 mol/L)
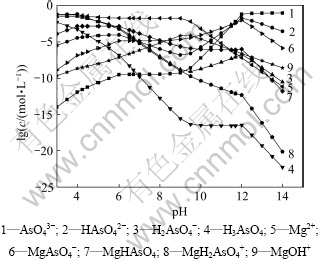
图4 [N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,各种离子分布随pH变化分布图
Fig.4 lg c-pH diagram for different ions of Mg-NH4-As-H2O system ([N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L)

图5 [N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,各种含砷离子百分比-pH图
Fig.5 Percentage of various arsenic-bearing ions versus pH ([N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L)
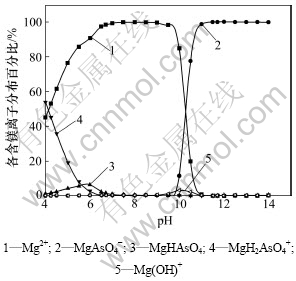
图6 [N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,各种含镁离子百分比-pH图
Fig.6 Percentage of various magnesium-bearing ions versus pH ([N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L)
3 实验验证
取100 mL钼酸铵溶液,其中钼的质量浓度为 96 g/L,砷的质量浓度为7.5 g/L,总氨浓度为5 mol/L,加入1.2倍理论量的MgCl2,在25 ℃搅拌1 h,控制反应终点pH为9左右,过滤,除砷后的钼酸铵溶液中镁和砷的质量浓度分别为0.51 g/L和9.5 mg/L,砷钼质量比为9.8×10-5。将理论计算值与实验值对比可以发现:理论计算除砷率为99.97%,实验中除砷率为99.87%,两者非常接近,除砷后溶液中砷钼质量比为9.8×10-5,可使得钼酸铵产品中砷含量不超标;溶液中残留的镁浓度与计算值较为接近,相对误差为5.8%,这可能是热力学计算过程中以浓度代替活度所导致。

图7 [N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,沉淀成分比例(摩尔比)与pH关系
Fig.7 Mole ratio of solid species at different pH of Mg-NH4-As-H2O system ([N] T=5 mol/L, [As]T=0.1 mol/L, [Mg]T=0.12 mol/L)
除砷后钼酸铵溶液中过量的镁,会在后续钼酸铵酸沉结晶时析出,使得产品中镁含量超标,因此,在酸沉结晶之前可用阳离子交换法除去溶液中多余的 镁[18]。
4 结论
(1) 应用同系线性规律估算出MgNH4AsO4溶度积,并且绘制出了25 ℃时,NH4-Mg-As-H2O系的热力学平衡图。
(2) 随着溶液中总氨浓度和镁盐用量的增大,平衡时砷含量更低。当溶液中总氨浓度[N]T=5 mol/L,[As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,整个pH范围内的沉淀稳定区如下:4.0<pH<6.0时为MgHAsO4的稳定区,6.0<pH<9.5时为MgNH4AsO4的稳定区,9.5<pH<11.7时为MgNH4AsO4和Mg(OH)2的稳定区,pH>11.7时为Mg(OH)2的稳定区。
(3) 当溶液中总氨浓度[N]T=5 mol/L,[As]T=0.1 mol/L, [Mg]T=0.12 mol/L,控制溶液的pH为9时,除砷效果最好,热力学上可将砷质量浓度除至2×10-6 g/L,溶液中残留的镁浓度为0.02 mol/L,且主要以Mg2+形态存在。
(4) 通过实验验证,理论计算结果与实验数据较为符合。当溶液中总氨浓度[N]T=5 mol/L,[As]T=0.1 mol/L, [Mg]T=0.12 mol/L时,镁平衡浓度相对误差为5.8%。
参考文献:
[1] Jiang S Y, Yang J H, Ling H F, et al. Extreme enrichment of polymetallic Ni-Mo-PGE-Au in Lower Cambrian black shales of South China: An Os isotope and PGE geochemical investigation[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2007, 254(1/2): 217-228.
[2] Kribek B, Sykorova I, Pasava J, et al. Organic geochemistry and petrology of barren and Mo-Ni-PGE mineralized marine black shales of the Lower Cambrian Niutitang Formation (Southern China)[J]. International Journal of Coal Geology, 2007, 72(3/4) 240-256.
[3] Orberger B, Vymazalova A, Wagner C, et al. Biogenic origin of inter-grown Mo sulfide and carbonaceous matter in Lower Cambrian black shales (Zunyi Formation, SouthernChina)[J]. Chemical Geology, 2007, 238(3/4): 213-231.
[4] 陈代雄, 唐美莲, 薛伟, 等. 高碳镍钼矿可选性试验研究[J]. 湖南有色金属, 2006, 22(6): 9-11.
CHEN Dai-xiong, TANG Mei-lian, XUE Wei, et al. Study on dressing of high carbon nickel-molybdenum ore[J]. Hunan Nonferrous Metals, 2006, 22(6): 9-11.
[5] 吴海国. 含碳镍钼矿提取镍钼冶炼新工艺实验研究[J]. 湖南有色金属, 2008, 24(2): 16-18.
WU Hai-guo. Ni-Mo carbon molybdenum smelting of nickel ore to test new technology research[J]. Hunan Nonferrous Metals, 2008, 24(2): 16-18.
[6] ZHAO Zhong-wei, ZHANG Gang, HUO Guang-sheng, et al. Kinetics of atmospheric leaching molybdenum from metalliferous black shales by air oxidation in alkali solution[J]. Hydrometallurgy, 2009, 97(3/4): 233-236.
[7] ZHAO Zhong-wei, YANG Liang, HUO Guang-sheng, et al. Solvent extraction of molybdenum blue from alkaline leaching solution of the Ni-Mo ore[J]. International Journal of Refractory Metals and Hard Materials, 2011, 29(2): 232-236.
[8] An B, Fu Z, Xiong Z,et al. Synthesis and characterization of a new class of polymeric ligand exchangers for selective removal of arsenate from drinking water[J]. Reactive & Functional Polymers, 2010, 70(8): 497-507.
[9] ZHAO You-cai, CHEN Jia-yong. Extraction of phosphorus, arsenic and/or silica from sodium tungstate and molybdate solutions with primary amine and tributyl phosphate as solvents. Ⅰ: Synergistic extraction and separation of phosphorus, arsenic and/or silica from tungstate and molybdate solutions[J]. Hydrometallurgy, 1996, 42(3): 313-324.
[10] 刘锐平,李星, 夏圣骥, 等. 高锰酸钾强化三氯化铁共沉降法去除亚砷酸盐的效能与机理[J]. 环境科学, 2005, 26(1): 72-75.
LIU Rui-ping, LI Xing, XAI Sheng-ji, et al. Effectiveness and mechanism of permanganate enhancing arsenite co-precipitation with ferric chloride[J]. Environmental Science, 2005, 26(1): 72-75.
[11] Masue Y K, Loeppert R H, Kramer T A. Arsenate and arsenite adsorption and desorption behavior on co-precipitated aluminum: Iron hydroxides[J]. Environ Sci Technol, 2007, 41(3): 837-842.
[12] 聂长明, 范明舫. 有机同系物性质的递变规律研究[J]. 有机化学, 2000, 20(1): 122-130.
NIE Chang-ming, FAN Ming-fang. Regularity of physicochemical properties for homologous organic compounds[J]. Chinese Journal of Organic Chemistry, 2000, 20(1): 122-130.
[13] Dean J A. Langep′s handbook of chemistry[M]. 15th ed. New York: Me Graw-Hill professional, 1999: 681-696.
[14] Warmadewanthi Liu J C. Recovery of phosphate and ammonium as struvite from semiconductor wastewater[J]. Separation and Purification Technology, 2009, 64(3): 368-373.
[15] Cornelis G, Poppeb S, Gervena T V, et al. Geochemical modelling of arsenic and selenium leaching in alkaline water treatment sludge from the production of non-ferrous metals[J]. Journal of Hazardous Materials, 2008, 159(2/3): 271-279.
[16] García-Lara A M, Montero-Ocampo C. Improvement of arsenic electro-removal from underground water by lowering the interference of other ions[J]. Water Air Soil Pollut, 2010, 205(1/2/3/4): 237-244.
[17] Udert K M, Larsen T A, Gujer W. Estimating the precipitation potential in urine-collecting systems[J]. Water Research, 2003, 37(11): 2667-2677.
[18] Huggins D K, Queneau P B, Ziegler R C, et al. Ion exchange purification of ammonium molybdate solutions[J]. Hydrometallurgy, 1980, 6(1/2): 63-73.
(编辑 杨幼平)
收稿日期:2011-05-03;修回日期:2011-07-06
基金项目:国家高技术研究发展计划(“863”计划)项目(2007AA06Z129);湖南有色集团-中南大学有色研究基金资助项目(Y2008-01-004)
通信作者:赵中伟(1966-),男,河北永年人,教授,从事冶金及功能材料方面的研究;电话:0731-88830476;E-mail: zhaozw@csu.edu.cn