J. Cent. South Univ. Technol. (2011) 18: 1897-1901
DOI: 10.1007/s11771-011-0920-2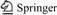
Inclusion behavior of oxybutynin with hydroxypropyl-β-cyclodextrin
ZHANG Pan-liang(张盼良)1, PAN Chun-yue(潘春跃)1, TANG Ke-wen(唐课文)2, LI Hong-jian(李洪建)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,Yueyang 414000, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Inclusion behavior of oxybutynin (OBN) with hydroxypropyl-β-cyclodextrin (HP-β-CD) was investigated by ultraviolet absorption spectrum and fluorescence spectrum. A reliable determination of the complex stoichiometry was provided by the continuous variation technique. Alcohol was added to further investigate the mechanism of the inclusion behavior. Thermodynamic constants ΔG, ΔH and ΔS for inclusion interaction of OBN and HP-β-CD were determined. The results show that host-guest complex with molar ratio of 1:1 is formed, and inclusion stability constant between OBN and HP-β-CD is 54.9 L/mol determined by ultraviolet spectrum and 11.1 L/mol determined by fluorescence spectrum. OBN has weak binding ability with HP-β-CD in aqueous solution (stability constant <102 L/mol) and addition of alcohol leads to a decrease of stability constant, which indicates that the hydrophobic force contributes to the inclusion process. ΔG, ΔH and ΔS are all less than zero, which indicates that the inclusion process is a spontaneous and exothermic process.
Key words: oxybutynin; β-cyclodextrin derivatives; inclusion interaction; ultraviolet spectrum; fluorescence spectrum
1 Introduction
Cyclodextrin (CD) and their derivatives are oligoglycosides with six (α-CD), seven (β-CD), eight (γ-CD) glucose units forming a ring with a cavity of moderate size. The interior of the ring is relatively hydrophobic, while the exterior is relatively hydrophilic, which enables them to form inclusion complexes with various guest molecules [1-2]. Several weak intermolecular forces between β-CDs and guest molecules, such as dipole–dipole, hydrophobic, van der Waals, electrostatic, and hydrogen bonding interaction, cooperatively contribute to the formation of the inclusion complexes [3]. Upon inclusion or partial inclusion of molecules within their hydrophobic interior, CDs can effectively shield the excited singlet state of molecules from the processes and enhance or weaken their ultraviolet absorption (or fluorescence) intensity [4]. Ultraviolet spectrum and fluorescence spectrum are two of the most common techniques used for the investigation of inclusion phenomena [5-7]. Applications of cyclodextrins and their derivatives cover various areas of study and industry, including the recognition of organic molecules [8], analytical chemistry [9], and the solubilization and stabilization of drugs [10-11].
Oxybutynin (4-(diethylamino)but-2-ynyl(RS)-2- cyclohexyl-2-hydroxy-2-phenylacetate, OBN) has been widely prescribed for the treatment of urinary tract disorders [12]. Separation of OBN enantiomers by high performance liquid chromalography (HPLC) with HP-β- CD as chiral mobile phase additive was investigated [13]. But the fundamental insight in the host-guest interaction between HP-β-CD and OBN was not provided. The inclusion interaction between OBN and HP-β-CD was investigated through ultraviolet absorption spectrum and fluorescence spectrum in this work. The inclusion stability constant and factors influencing the inclusion process and thermodynamic constants for inclusion reaction were determined.
2 Experimental
2.1 Materials and equipment
Oxybutynin (OBN, racemate, purity≥99.5%) was purchased from Hengshuo Pharmaceutical Chemical Co. Inc. (Hubei Province, China). Hydroxypropyl-β- cyclodextrin (HP-β-CD) was bought from Qianhui Fine Chemical & Co. Inc. (Shandong China). All other chemicals were of analytical-reagent grade.
Ultraviolet (UV) absorption spectral measurements were carried out with a TU-1901 double-beam UV-Vis spectrophotometer (Beijing Purkinje General Instrument Co. Ltd., Beijing, China), and fluorescence measurements were performed with a fluorescence spectrophotometer (Perkin-Elmer Inc., USA).
2.2 Determination of ultraviolet absorption spectrum of OBN and HP-β-CD
OBN was firstly dissolved in alcohol and then diluted by deionized water and HP-β-CD was dissolved in deionized water. The solution of OBN and the solution of HP-β-CD were subsequently transferred into a 1 cm × 1 cm quartz cell to record UV absorption spectrum. The measurement of absorption was made against a blank solution treated in the same way but without solute.
2.3 Determination of stoichiometry
OBN and HP-β-CD were dissolved in a 50:50 (v/v, volume fraction) mixture of alcohol and water at various molar ratios (1:9-9:1) and stirred to equilibrium for 12 h at room temperature. UV absorbance changes between free and complex OBN were measured at 223 nm and the stoichiometry was determined.
2.4 Determination of inclusion stability constant by UV absorption spectrum
The OBN stock solution was prepared by dissolving the OBN in alcohol with a concentration of 1×10-3 mol/L. 1 mL of OBN stock solution was then transferred into a 10 mL volumetric burette and then an appropriate amount of 0.1 mol/L HP-β-CD solution and 2 mL of K2HPO4/KH2PO4 (0.1 mol/L, pH=6.9) buffer solution were added. The solutions were diluted to a final volume of 10 mL with an increasing concentration of HP-β-CD (0-30×10-3 mol/L). The final solution was mixed thoroughly under ultrasonic for 30 min, and then equilibrated for 12 h. The working solution was transferred into a 1 cm × 1 cm quartz cell to record absorption spectrum. The measurement of absorption was carried out against a blank solution treated in the same way but without solute.
2.5 Determination of fluorescence spectrum of OBN
OBN was firstly dissolved in alcohol and then diluted by deionized water. The solution was subsequently transferred into a 1 cm × 1 cm quartz cell to record fluorescence spectrum. The excitation slit was set at 15 nm and the emission slit was set at 5 nm.
2.6 Determination of inclusion stability constant by fluorescence spectrum
The OBN stock solution was prepared by dissolving the OBN in alcohol with a concentration of 1×10-3 mol/L. 1 mL of OBN stock solution was then transferred into a 10 mL volumetric burette and an appropriate amount of 0.1 mol/L HP-β-CD solution and 2 mL of K2HPO4/ H3PO4 (0.1 mol/L, pH=6.9) buffer solution were added. The solutions were diluted to a final volume of 10 mL with an increasing concentration of HP-β-CD (0-70× 10-3 mol/L). The final solution was mixed thoroughly under ultrasonic for 30 min, and then equilibrated for 12 h in a water bath at a fixed temperature. The working solution was transferred into a 1 cm × 1 cm quartz cell to record fluorescence spectrum. The determination was carried out in solvents of different concentrations of alcohol and at different temperatures.
3 Results and discussion
3.1 Ultraviolet absorption spectra of OBN and HP-β- CD
The ultraviolet absorption spectra of OBN and HP-β-CD are shown in Fig.1. In the wavelength range from 200 nm to 400 nm of the absorption spectrum of OBN, a shoulder peak appears at 223 nm while the absorbance of HP-β-CD is quite weak in the same wavelength range. Compared to OBN, the absorption of HP-β-CD can be neglected here.

Fig.1 UV absorption spectra in condition of c(OBN)=1×10-4 mol/L and c(HP-β-CD)=0.03 mol/L
3.2 Stoichiometry between OBN and HP-β-CD
Before proceeding with the calculation of the inclusion stability constant, it is important to determine the stoichiometry of the complexes. A reliable determination of the complex stoichiometry is provided by the continuous variation technique (Job’s plot) [14]. The continuous variation plot of the UV absorbance changes between free and complex OBN (Fig.2) shows that the enhancement of absorbance is maximal when the molar ratio of OBN to HP-β-CD is 1:1. This indicates that a complex with a 1:1 stoichiometry is predominant in the solution.
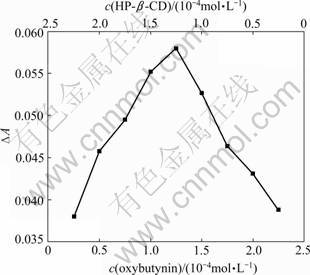
Fig.2 Continuous variation plot for OBN-HP-β-CD system
3.3 Determination of inclusion stability constant by UV absorption spectrum
The UV absorption spectra of OBN in different concentrations of HP-β-CD are shown in Fig.3. With the increase of the HP-β-CD concentration, the shoulder peak is slightly blue shifted with a gradual increase in absorbance, which indicates that inclusion interaction occurs between HP-β-CD (host) and OBN (guest). This behavior may be attributed to the inductive effect occurring between the valence electron of the guest and the high electron density in the ring interior of the host.
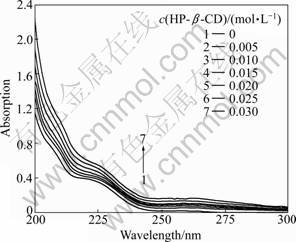
Fig.3 Influence of HP-β-CD concentration on ultraviolet spectra of OBN (Temperature: ambient temperature)
UV absorbance changes between free and complex OBN at 223 nm were chosen for the calculation of inclusion stability constants. Since 1:1 host–guest type inclusion complex forms between HP-β-CD and OBN, following equations can be obtained:
KS
H+G HG (1)
HG (1)
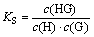 (2)
(2)
where H represents the host HP-β-CD and G represents the guest OBN, respectively.
Benesi–Hildebrand relation for 1:1 inclusion complex can be given as follows [14]:
 (3)
(3)
where ΔA is the absorption difference of OBN in the presence and absence of HP-β-CD; Δε is the molar absorption coefficient difference between free and complexed OBN; KS is the inclusion stability constants.
Figure 4 depicts the plot of 1/ΔA vs 1/c(H) and good linear correlation is obtained, which further confirms the formation of a 1:1 inclusion complex. From the intercept and slope values of this plot, KS is evaluated as 54.9 L/mol.
3.4 Fluorescence spectrum of OBN
Figure 5 shows the fluorescence spectra of OBN. The maximum excitation and emission wavelengths are at 250 nm and 310 nm, respectively.
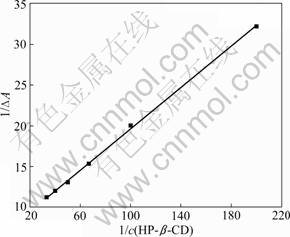
Fig.4 Double reciprocal linear fitting of 1/ΔA vs 1/c(HP-β-CD)

Fig.5 Maximum excitation wavelength and maximum emission wavelength of OBN
3.5 Determination of inclusion stability constant by fluorescence spectra
Figure 6 shows that adding HP-β-CD to OBN solution results in a regular change of the fluorescence spectra. These results suggest that the inclusion complex is formed between HP-β-CD and OBN. With the increase of HP-β-CD concentration, the peak at 310 nm disappears gradually while a new peak appears gradually at 360 nm. There is a clear isosbestic point observed at 318 nm. It is also observed from Fig.6 that the fluorescence is enhanced considerably after HP-β-CD is added. The HP-β-CD cavity provides an apolar environment for the OBN molecule and the motion of the OBN molecule in the cavity is largely confined. Thus, the enhanced rigidity of the OBN molecule results in an increase of its fluorescence quantum yield [5].
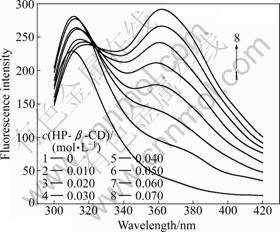
Fig.6 Influence of HP-β-CD concentration on fluorescence spectra of OBN (Temperature: 35 °C)
For the formation of a 1:1 complex, inclusion stability constant can be calculated based on the Benesi–Hildebrand plots using fluorescence data [4-5]:
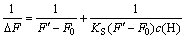 (4)
(4)
where c(H) represents the initial concentration of HP-β-CD; ΔF is the florescence intensity difference of OBN in the presence and absence of HP-β-CD; F0 is florescence intensity of OBN in the absence of HP-β-CD. F′ is limiting intensity of florescence; KS is the inclusion stability constant. The plot of 1/?F vs 1/c(H) yields a straight line (Fig.7). From the plot, KS is evaluated as 11.1 L/mol. The inclusion stability constant determined by florescence intensities is different from that determined by UV absorption spectrum. The possible reason for this may be that measurement errors exist between the two measuring methods and/or the temperature is set differently from each other.
3.6 Influence of alcohol content in solvent on inclusion reaction
The influence of alcohol content in solvent on inclusion stability constant was investigated by fluorescence spectra to further understand the driving force of the inclusion reaction. Figure 8 shows that the inclusion stability constant decreases with the increase of alcohol content in solvent, which indicates that hydrophobic interaction is the driving force of the inclusion interaction. The increase of alcohol content will weaken the driving force and lead to a decrease of inclusion stability constant.
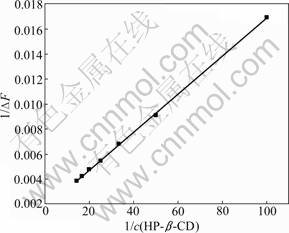
Fig.7 Linear fitting of 1/ΔF vs 1/c(HP-β-CD)

Fig.8 Influence of ethanol content on inclusion stability constant (KS)
3.7 Determination of thermodynamic parameters of inclusion process
According to the chemical structures of OBN and HP-β-CD, it is assumed that the molecular interactions such as dipole–dipole, hydrophobic, van der Waals and hydrogen bonding interaction cooperatively contribute to the inclusion process. The thermodynamic parameters of dynamic equilibrium inclusion process are determined based on the inclusion stability constants at different temperatures. The relation between inclusion stability constant and thermodynamic temperature can be given by Van’t Hoff equation (Eq.(5)) [15] and the free energy change can be calculated from inclusion stability constant [16]:
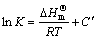 (5)
(5)
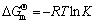 (6)
(6)
where 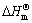 is the standard enthalpy change; R is the universal gas constant; C′ is a constant.
is the standard enthalpy change; R is the universal gas constant; C′ is a constant.
The plot of ln K vs 1/T yields a straight line and enthalpy change ΔH and entropy change ΔS can be calculated from the slop and the intercept of the plot, respectively. All the thermodynamic parameters are given in Table 1.
Table 1 Stability constants and thermodynamic parameters for binding of OBN and HP-β-CD at different temperatures

It is observed from Table 1 that, 1) Decrease of the temperature will enhance the inclusion interaction of OBN and HP-β-CD; 2) ΔG<0 indicates the inclusion reaction is a spontaneous process; 3) ΔH<0 and ΔS<0 indicate that the inclusion reaction is a exothermic reaction. The van der Waals interaction and the release of high-energy water molecule from the ring of the host molecule result in the negative enthalpy change. The steric hindrance caused by the geometric shape of host/guest molecules and the restriction of the translational and rotational degree of freedom result in the negative entropy change [16].
4 Conclusions
1) OBN and HP-β-CD can form a inclusion complex with a 1:1 stoichiometry.
2) The addition of alcohol into the solvent will lead to a decrease of the inclusion stability constant.
3) The thermodynamic parameters ΔG, ΔH and ΔS are all less than zero, which indicates the inclusion process is a spontaneous and exothermic process.
4) Ultraviolet absorption spectrum and fluorescence spectrum both can be used to investigate the inclusion interaction between OBN and HP-β-CD. The difference between the measurement results of the two methods may result from the different mechanisms of ultraviolet absorption and fluorescence emission and/or from the different experimental conditions.
References
[1] MOHANTY J, BHASIKUTTAN A C, NAU W M, PAL H. Host-guest complexation of neutral red with macrocyclic host molecules: Contrasting pKa shifts and binding affinities for cucurbit[7]uril and β-cyclodextrin [J]. Journal of Physical Chemistry B, 2006, 110(10): 5132-5138.
[2] JORDHEIM L P, DEGOBERT G, DIAB R, PEYROTTE S, PERIGAUD C, DUMONTET C, FESSI H. Inclusion complexes of a nucleotide analogue with hydroxypropyl-beta-cyclodextrin [J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2009, 63(1/2): 11-16.
[3] REKHARSKY M V, INOUE Y I. Complexation thermodynamics of cyclodextrins [J]. Chemical Reviews, 1998, 98(5): 1875-1918.
[4] SHANMUGAM M, RAMESH D, NAGALAKSHMI V, KAVITHA R, RAJAMOHAN R, STALIN T. Host–guest interaction of l-tyrosine with-cyclodextrin [J]. Spectrochim Acta Part A, 2008, 71(1): 125-132.
[5] LI Jin-xia, ZHAO Chun, CHAO Jian-bin. Investigation on the inclusion behavior of norfloxacin with 2-methyl-β-cyclodextrin [J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2008, 62(3/4): 325-331.
[6] CRUPI V, FICARRA R, GUARDO M, MAJOLINO D, STANCANELLI R, VENUTI V. UV–vis and FTIR–ATR spectroscopic techniques to study the inclusion complexes of genistein with β-cyclodextrins [J]. Journal of Pharmaceutical and Biomedical Analysis, 2007, 44(1): 110-117.
[7] LIU Yu, LI Xiao-yun, ZHANG Heng-yi, LI Chun-ju, DING Fei. Cyclodextrin-driven movement of cucurbit[7]uril [J]. Journal of Organic Chemistry, 2007, 72(10): 3640-3645.
[8] ZHOU Shan-shan, OUYANG Jin, BAEYENS WRG, ZHAO Hui-chun, YANG Yi-ping. Chiral separation of four fluoroquinolone compounds using capillary electrophoresis with hydroxypropyl-β- cyclodextrin as chiral selector [J]. Journal Chromatography A, 2006, 1130(2): 296-301.
[9] AMEYIBOR E, STEWART J T. Enantiomeric HPLC Separation of Selected Chiral Drugs Using Native and Derivatized β-Cyclodextrins as Chiral Mobile Phase Additives [J]. Journal of Liquid Chromatography & Related Technologies, 1997, 20(6): 855-869.
[10] POLYAKOVE N E, LESHINA T V, HAND E O, PETRENKO A, KISPERT L D. β-Ionone cyclodextrins inclusion complexes: 1H-NMR study and photolysis [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2004, 161(2/3): 261-267.
[11] AL-MARZOUQIL A H, SHEHATTA I, JOBEL B, DOWAIDAR A. Phase solubility and inclusion complex of itraconazole with β-cyclodextrin using supercritical carbon dioxide [J]. Journal of Pharmaceutical Sciences, 2006, 95(2): 292-304.
[12] RECUERO V, FERRERO M, GOTOR-FERNANDEZ V, BRIEVA R, GOTOR V. Enzymatic resolution of hindered cyanohydrins, key precursors of muscarinic receptor antagonists [J]. Tetrahedron: Asymmetry, 2007, 18(8): 994-1002.
[13] GUO Na, GAO Xin-xing, XU Guo-fang, GUO Xing-jie. High performance liquid chromatographic separation of oxybutynin enantiomers using chiral mobile phase additive [J]. Chinese Journal of Chromatography, 2008, 26(2): 259-261. (in Chinese)
[14] PARK K, KIM K H, JUNG S, LIM H, HONG C, KANG J. Enantioselective stabilization of inclusion complexes of metoprolol in carboxymethylated β-cyclodextrin [J]. Journal of Pharmaceutical and Biomedical Analysis, 2002, 27(3/4): 569-576.
[15] CALABRO M L, TOMMASINI S, DONATO P, RANERI D, STANCANELLI R, FICARRA P, FICARRA R, COSTA C, CATANIA S, RUSTICHELLI C, GAMBERINI G. Effects of α- and β-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3-hydroxyflavones [J]. Journal of Pharmaceutical and Biomedical Analysis, 2004, 35(2): 365-377.
[16] BETTINETTI G P, MURA P, LIGUORI A, BRAMANTI G, GIORDANO F. Solubilization and interaction of naproxen with cyclodextrins in aqueous solution and in the solid state [J]. Farmaco Prat, 1988, 43(11): 331-343.
(Edited by HE Yun-bin)
Foundation item: Project(20976041) supported by the National Natural Science Foundation of China
Received date: 2010-10-01; Accepted date: 2011-02-05
Corresponding author: TANG Ke-wen, Professor, PhD; Tel: +86-730-8640921; E-mail: tankewen@sina.com