J. Cent. South Univ. (2012) 19: 41-45
DOI: 10.1007/s11771-012-0970-0
High-performance porous carbon for supercapacitors prepared by one-step pyrolysis of PF/gelatin blends
YI Bin(易斌)1, CHEN Xiao-hua(陈小华)1, LIU Yun-quan(刘云泉)1, GUO Kai-min(郭凯敏)2,
CHEN Chuan-sheng(陈传盛)2, ZENG Bin(曾斌)1, LONG Hui(龙慧)1
1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. College of Physics and Electronic Science,
Changsha University of Science and Technology, Changsha 410114, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: A high-performance porous carbon material for supercapacitor electrodes was prepared by using a polymer blend method. Phenol-formaldehyde resin and gelatin were used as carbon precursor polymer and pore former polymer, respectively. The blends were carbonized at 800 °C in nitrogen. SEM, BET measurement and BJH method reveal that the obtained carbon possesses a mesoporous characteristic, with the average pore size between 3.0 nm and 5.0 nm. The electrochemical properties of supercapacitor using these carbons as electrode material were investigated by cyclic voltammetry and constant current charge-discharge. The results indicate that the composition of blended polymers has a strong effect on the specific capacitance. When the mass ratio of PF to gelatin is kept at 1:1, the largest surface area of 222 m2/g is obtained, and the specific capacitance reaches 161 F/g.
Key words: mesoporous carbon; polymer blend; supercapacitor; pseudocapacitance; electrochemical properties
1 Introduction
Electrochemical capacitors (often called super- capacitors) [1-2] are promising power sources for portable systems and automotive applications. They are generally classified into two categories depending on the charge-storage mechanism. The energy storage mechanism in electric double-layer capacitors (EDLCs) is based on an electrostatic attraction between charges along the double layer, formed at the electrode/ electrolyte interface. For the capacitors in the other class, called pseudocapacitors, the storage mechanism in this case is related to fast faradaic redox reactions.
In EDLCs, mostly based on activated carbons (ACs) [3-5] and considering that only the charging of the double-layer is involved, it should be expected that the higher the specific surface area of an activated carbon is, the higher the capacitance is. However, observing the low values of specific capacitance which were reported in Refs. [6-7] for activated carbons with surface areas ranging up to 2 000-3 000 m2/g, one can assume that not all the pores are effective in the charge accumulation. For example, it has been demonstrated that very narrow microspores do not contribute to the total double layer capacitance because of a molecular sieving effect [8-9]. Polymer blend technique [10-14] enables us to tailor the diameter of the obtained porous carbon to form mesopores by controlling the blending ratio of both polymers. The formed mesopores can provide more favorable and quick pathway for ion penetration; however, they have to be implemented at expense of scarifying the specific surface area of the porous carbon. The low specific surface area limits their application as supercapacitor electrode material.
Although a high specific surface area is regarded as a primary requirement for carbon electrodes to be used in EDLCs, some other aspects of the surface physico-chemical properties can be critical to the electrochemical performance. Therefore, materials that present pseudocapacitance properties are promising alternatives to EDLCs. In this case, high capacitance properties could be demonstrated by low surface area carbon electrodes. There is a possibility that heteroatom such as oxygen can induce pseudocapacitive effects [15-18]. RAYMUNDO-PI?ERO et al [19] have reported a carbonaceous material for supercapacitors obtained by carbonization of a seaweed biopolymer. Although the material developed has a low specific surface area (270 m2/g), the capacitance value per mass of active material (200 F/g) is comparable to the best activated carbons, due to the oxygen present in the carbon network participating in pseudofaradic charge-transfer reactions. The drawback is that the pore sizes cannot be controlled effectively.
Our strategy was to prepare a mesoporous carbon material containing a high concentration of functional groups by using a polymer blend method. Phenol- formaldehyde resin was chosen as carbon precursor polymer and oxygen-rich gelatin was chosen as pore former polymer. Pores can be controlled by changing PF-to-gelatin mass ratios, and a high amount of oxygen is incorporated into the carbon framework. Electrochemical properties of the obtained porous carbons as supercapacitor electrodes were evaluated.
2 Experimental
2.1 Materials
The gelatin was purchased from Sinopharm Chemical Reagment Co., Ltd. (Shanghai, China). A Novolac-type phenol-formaldehyde polymer (PF) was produced by Changsha Zhida Chemical Ind. Co. Hexamethylenetetramine was purchased from Shantou Xilong Chemical Factory, Guangdong Province, China. These chemicals were used directly without further purification.
2.2 Preparation and characterization of porous carbon
The porous carbon was prepared in the following way. Gelatin was dissolved in water at 60 °C, and PF resins were dissolved in ethanol solution including 10% (mass fraction) of hexamethylenetetramine (cross-linking agent) against the mass of PF resin. Then, a PF/gelatin polymer blend solution was formed by the addition of PF resin into gelatin solution under continuous stirring. Subsequently, the blends were cured at 180 °C for 5 h in a dynamic vacuum. The blend ratios of PF to gelatin were controlled through varying the relative mass ratios of PF to gelatin as 4:1, 2:1, 1:1, 1:2 and 1:4. The blends were finally carbonized at 800 °C for 1 h under a nitrogen atmosphere. The porous carbons obtained were denoted as PG-1, PG-2, PG-3, PG-4 and PG-5.
The temperature of the carbonization of the blends was determined by thermogravimetric analysis (TGA) using a DT-40 Shimadzu thermal analyzer with a rate of 10 °C/min from room temperature to 900 °C at a nitrogen flow. The morphology of the sample was observed on a Hitachi S-4800 scanning electron microscope (SEM). Elemental analysis of the sample was provided by energy dispersive X-ray (EDX). Quantachrome measuring instrument (NOVA2000) was employed to investigate the pore properties of the samples. The specific surface area was calculated using the Brunauere-Emmette-Teller (BET) equation. The pore size distributions were obtained from the adsorption branch of the nitrogen isotherms by Barrette-Joynere- Halenda (BJH) method. Finally, pore volumes were estimated to be the liquid volume of adsorption (N2) at a relative pressure of 0.98.
2.3 Preparation and property measurements of porous carbon electrode
A mixture of porous material, carbon black and PTFE at a mass ratio of 80:10:10 was pressed onto a foam nickel followed by pressing the composite in an extrusion machine. At last, the electrode was dried at 120 °C for 12 h in a vacuum oven before use. Cyclic voltagrammetry was carried out on an electrochemical workstation (CHI-660b, China) in 6 mol/L KOH solution. The capacitive behavior of the composite was investigated by constant current charge-discharge in Land system (Land, CT-2001A).
3 Results and discussion
Figure 1 shows TG curves of PF, gelatin and polymer blend (PF-to-gelatin mass ratio of 1:1) in nitrogen atmosphere. After heating to 900 °C, the carbon yields in PF and gelatin are about 58% and 18%, respectively. PF shows a gradual mass loss at 250-600 °C and gelatin shows a rapid mass loss at 200-400 °C. The TG curve of the polymer blend coincides roughly with that deduced from the PF, gelatin and the blend ratio. Based on the TG curves, 800 °C is chosen to be the pyrolysis temperature, because at this temperature most of the gelatin has been decomposed whereas the mass of the blends has not decreased significantly.

Fig. 1 TG curves of PF, gelatin and their blend (1:1) in nitrogen atmosphere
As shown in Fig. 2(a), the sample before pyrolysis apparently does not have any pores on their surface. As shown in Fig. 2(b), after pyrolysis, the surface of the PG-3 becomes rough, and many pores appear in the carbon matrix. Macropores and mesopores due to the evolution of decomposition gases are visible, and induced porosity of the electrodes should be favorable for electrolyte penetration.
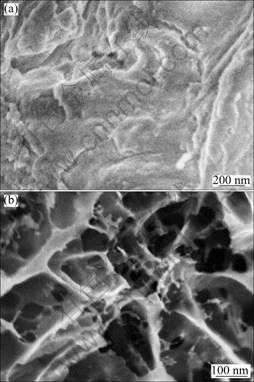
Fig. 2 SEM images of PG-3: (a) Before pyrolysis, (b) After pyrolysis at 800 °C
The pore size distributions and the pore texture properties of the samples are shown in Fig. 3 and Table 1. It can be seen that all resultant carbon exhibits a mesoporous structure. PG-3 has the highest BET surface area of 222 m2/g, and a total pore volume of 0.165 cm3/g measured at a relative pressure of 0.98, most of the pores being mesopores with an average pore diameter of 3.0 nm, which are the valid pores for EDLC electrode material [20]. In the meantime, PG-5 exhibits the lowest BET surface area of 89 m2/g, with a much lower total pore volume of 0.112 cm3/g and an average pore diameter of 5.0 nm. It is obvious that the SBET increases from 93 m2/g of PG-1 to 222 m2/g of PG-3, then decreases to 89 m2/g of PG-5 with increasing the PF-to-gelatin ratio. The composition of blended polymers shows a strong effect on the resultant surface area.
Figure 4 presents cyclic voltammograms (CVs) of the PG-3(1:1) electrode at different scan rates. It can be found that the current increases with the increase of the scan rate. At 2 mV/s or 5 mV/s, a rectangular shape can be observed. At 10 mV/s, the shape of the rectangle is slightly distorted. At 25 mV/s or 50 mV/s, the shape is clearly distorted, which is due to the resistance of ion migration in pores because the increase of the scan rate aggravates the delay of the current to reach a horizontal value after reversal of the potential scan. Similar behaviors have been observed for PG-1, PG-2, PG-4 and PG-5. Specific capacitances (CSP) evaluated from the CV curves are summarized in Table 1. It can be seen that the CSP passes through a maximum with the change of PF-to-gelatin ratios from 4:1 to 1:4. The highest specific capacitance of 161 F/g is for the PG-3 with the PF-to-gelatin ratio of 1:1. The relatively high specific capacitance is resulted from low specific surface area, indicating that well-developed mesoporous structure plays an important role in outstanding electrochemical performances.
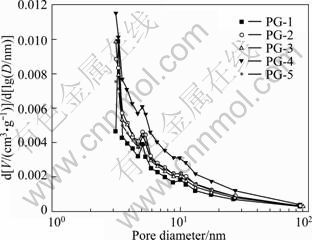
Fig. 3 Pore size distributions of porous carbon
Table 1 Pore texture properties and specific capacitance of porous carbon
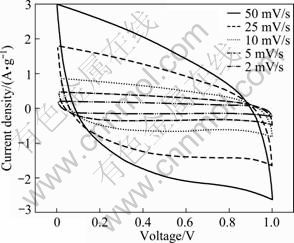
Fig. 4 CVs of PG-3 electrode at different scan rates
The chronopotentiogram of PG-3 electrode at different charge-discharge currents is shown in Fig. 5. The regular shape of the curves indicates that the cell presents a reversible charge-discharge process and that the stability of the capacitor is good. Another noticeable feature of the charge-discharge curves is a sudden drop of potential at the beginning stage of discharge, which is associated with the resistance of the cell. The good linear relationship between the voltage and the time with increasing the current density indicates that the porous carbon displays excellent capacitive characteristics.
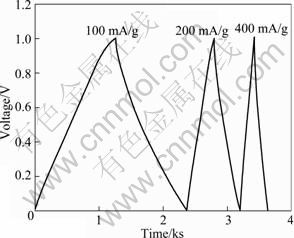
Fig. 5 Charge-discharge curves of PG-3 electrode at different current densities
The excellent electrochemical performance of porous carbon could be ascribed to the following characteristics. Firstly, according to SHI’s theory [21], for most carbon materials, the macropores contribution to total surface area is negligible (usually less than 2 m2/g) compared to that from mesopores and micropores. In addition, the total surface area is also conventionally divided into two parts: the micropore surface area and the external surface area (all of the surface area excluding the micropore surface area). For most carbons, the external surface area is equivalent to the mesopore surface area. Instead of using one fixed double layer capacitance for all porous surfaces, it is reasonable to assume that the double layer capacitance per unit micropore area (Cmic) is different from that per unit external surface (Cext). On the basis of above discussion, the specific capacitance (C) can be written as the sum of two different parts:
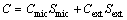 (1)
(1)
where Smic and Sext are the micropore surface area and external surface area, respectively. Parameters Cmic and Cext are respectively 0.195 and 0.74 F/m for activated microbeads. Consequently, one may assume that the porous carbon obtained has a high Sext in order to demonstrate high capacitance by low specific surface area.
The oxygen functional group is another important factor. According to the elemental analysis, as listed in Table 2, PG-3 is constituted mainly by element carbon (82.53%, molar fraction) and oxygen (17.33%, molar fraction). A small amount of Cl (0.14%, molar fraction) is contributed to the impurity of gelatin. The initial oxygen content of gelatin ranges between 30% and 40% (molar fraction). The results indicate that a high amount of oxygen is retained in the carbon framework after carbonization.
Table 2 Elemental analysis of PG-3

Based on the concept of polymer-blending, neutral gelatin nature polymer with lots of amide groups can be readily blended with commercially available PF resin through multiple hydrogen bonding between hydroxyl groups (-OH) or hydroxylmethylene groups (-CH2OH) in the PF polymer. After pyrolysis of the PF/gelatin blends, most of the gelatin is decomposed, forming mesopores which provide more favorable and quick pathway for ion penetration. At the same time, an appreciable amount of oxygen is incorporated into the carbon matrix. Many types of oxygen-containing functional groups such as phenolic hydroxyl groups, carboxyl groups and carbonyl groups could form on the surface of porous carbon. The presence of oxygenated groups on the surface of the porous carbon can affect the capacitance of the materials mainly in two different ways: 1) The oxygenated groups can improve the wettability of the carbon surface, which is very important to maximize the access of the electrolyte to the surface of carbon; 2) The presence of oxygenated groups causes the double-layer capacitance to arise from quick faradaic charge transfer reactions (pseudocapacitance) as well as from electrostatic charging.
It is predicted that the phenolic hydroxyl group, which has a weaker polarity on oxygen-containing functional groups than the carboxyl group, might produce a change bias, but no catalytic effect, thus enhancing the formation of an electric double-layer (EDL) according to the following equation:
C—OHδ-+K+ C—OH//K+ (2)
C—OH//K+ (2)
where K+ indicates cation and the symbol “//” indicates the adsorbed state by EDL.
It can be assumed that a well-adjusted balance of PF and gelatin is required to obtain an optimal performance. The content of gelatin must be high enough to favor a large gas evolution, which develops porosity, and to obtain the largest amount of residual oxygen, which contributes to the pseudocapacitance. The discussion above explains that PG-3 presents the largest specific capacitance, and although the surface area of the obtained porous carbon is limited, the specific capacitance is remarkable.
4 Conclusions
1) Porous carbons were prepared by one-step carbonization of PF/gelatin blends without any additional activation process, and were used as electrode materials for supercapacitors.
2) The obtained porous carbons exhibit mesoporous structures with an average pore diameter of 3-5 nm. Although the porous carbons have a relatively lower specific surface area, they present large specific capacitance (up to 161 F/g), which is associated with their mesoporous structures and pseudocapacitive effect.
3) The blend ratio of PF to gelatin plays an important role in controlling the nanostructure and electrochemical capacitance of porous carbons. This new generation of pseudocapacitive mesoporous carbons could be very promising for advanced capacitors due to the high capacitance properties, low cost and simple process.
References
[1] WANG Da-wei, LI Feng, LIU Min. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage [J]. Angewandte Chemie, 2007, 120(2): 379-382.
[2] FANG Jing, CUI Mu, LU Hai. Hybrid supercapacitor based on polyaniline doped with lithium salt and activated carbon electrodes [J]. Journal of Central South University of Technology, 2009, 16(3): 434-439.
[3] KIM Y J, HORIE Y, MATSUZAWA Y. Structural features necessary to obtain a high specific capacitance in electric double layer capacitors [J]. Carbon, 2004, 42(12/13): 2423-2432.
[4] XU Bin, WU Feng, CHEN Ren-jie. Mesoporous activated carbon fiber as electrode material for high-performance electrochemical double layer capacitors with ionic liquid electrolyte [J]. Journal of Power Sources, 2010, 195(7): 2118-2124.
[5] LIU Ye-xiang, LI Jing, LAI Yan-qing. Preparation and properties of pitch carbon based supercapacitor [J]. Journal of Central South University of Technology, 2007, 14(5): 601-606.
[6] LOZANO-CASTELLO D, CAZORLA-AMOROS D, LINARES- SOLANO A. Influence of pore structure and surface chemistry on electric double layer capacitance in non-aqueous electrolyte [J]. Carbon, 2003, 41(9): 1765-1775.
[7] QIAO W, YOON S H, MOCHIDA I. KOH activation of needle coke to develop activated carbons for high-performance EDLC [J]. Energy & Fuels, 2006, 20(4): 1680-1684.
[8] SALITRA G, SOFFER A, ELIAD L. Carbon electrodes for double-layer capacitors I. Relations between ion and pore dimensions [J]. Journal of the Electrochemical Society, 2000, 147: 2486-2493.
[9] ELIAD L, SALITRA G, SOFFER A. Ion sieving effects in the electrical double layer of porous carbon electrodes: Estimating effective ion size in electrolytic solutions [J]. J Phys Chem B, 2001, 105(29): 6880-6887.
[10] CHANG Kai-wen, LIM Zheng-yi, DU Fang-yi. Synthesis of mesoporous carbon by using polymer blend as template for the high power supercapacitor [J]. Diamond and Related Materials, 2009, 18(2/3): 448-451.
[11] PATEL N, OKABE K, OYA A. Designing carbon materials with unique shapes using polymer blending and coating techniques [J]. Carbon, 2002, 40(3): 315-320.
[12] KATSUYA F, YOSHIKIYO H, TATSURO H. Estimation of pore structures in carbon fibers prepared from polymer blends during carbonization by small-angle X-ray scattering [J]. Carbon, 2008, 46(4): 722.
[13] SUBRAMANIA A, KALYANA SUNDARAM N T, VIJAYA KUMAR G. Structural and electrochemical properties of micro-porous polymer blend electrolytes based on PVdF-co-HFP- PAN for Li-ion battery applications [J]. Journal of Power Sources, 2006, 153(1): 177-182.
[14] YAMAZAKI M, KAYAMA M, IKEDA K. Nanostructured carbonaceous material with continuous pores obtained from reaction-induced phase separation of miscible polymer blends [J]. Carbon, 2004, 42(8/9): 1641-1649.
[15] DENISA H J, MYKOLA S, GAO Q L. Combined effect of nitrogen- and oxygen-containing functional group of microporous activated carbon on its electrochemical performance in supercapacitors [J]. Advanced Functional Materials, 2009, 19(3): 438-447.
[16] ODA H, YAMASHITA A, MINOURA S. Modification of the oxygen-containing functional group on activated carbon fiber in electrodes of an electric double-layer capacitor [J]. Journal of Power Sources, 2006, 158(2): 1510-1516.
[17] CENTENO T A, STOECKLI F. The role of textural characteristics and oxygen-containing surface groups in the supercapacitor performances of activated carbons [J]. Electrochimica Acta, 2006, 52(2): 560-566.
[18] ZHANG Chuan-xiang, LONG Dong-hui, XING Bao-lin. The superior electrochemical performance of oxygen-rich activated carbons prepared from bituminous coal [J]. Electrochemistry Communications, 2008, 10(11): 1809-1811.
[19]  E, LEROUX F, BEGUIN F. A high performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer [J]. Advanced Materials, 2006, 18(14): 1877-1882.
E, LEROUX F, BEGUIN F. A high performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer [J]. Advanced Materials, 2006, 18(14): 1877-1882.
[20] MORIGUCHI I, NAKAWARA F, YAMADA H. Electrical double-layer capacitive properties of colloidal crystal templated nanoporous carbons [J]. Stud Surf Sci Catal, 2005, 156: 589-594.
[21] SHI H. Activated carbons and double layer capacitance [J]. Electrochimica Acta, 1996, 41(10): 1633-1639.
(Edited by YANG Bing)
Foundation item: Projects(50772033, 50972043) supported by the National Natural Science Foundation of China; Project(09JJ3095) supported by the Natural Science Foundation of Hunan Province, China; Project(09A001) supported by the Scientific Research Fund of Hunan Provincial Education Department, China; Project(2010FJ3151) supported by the Science and Research Plan of Hunan Province, China; Project supported by the Science and Technology Innovative Research Team in Higher Education Institution of Hunan Province, China
Received date: 2010-12-16; Accepted date: 2011-04-07
Corresponding author: CHEN Xiao-hua, Professor; Tel: +86-731-88822663; E-mail: enyage@sohu.com