基于液晶模板的蒸发诱导自组装技术 合成高催化活性的有序介孔氧化钛
来源期刊:中国有色金属学报(英文版)2014年第4期
论文作者:刘 晨 李佑稷 徐 鹏 李泽时 曾孟雄
文章页码:1072 - 1078
关键词:二氧化钛;有序介孔;液晶模板;亚甲基蓝;降解
Key words:titania; ordered mesopore; liquid crystal template; methylene blue; degradation
摘 要:利用蒸发诱导自组装技术,用液晶为模板制备有序介孔氧化钛(OMPT),探讨影响亚甲基蓝(MB)氧化降解效率的主要因素,包括MB的初始浓度、pH值和催化剂浓度。结果表明,所获得的OMPT具有二维六方介孔结构,粒径小,比表面积大,表现出高的热稳定性,这些都导致其比催化剂P25和溶胶–凝胶法制备的纳米氧化钛颗粒(NPT)有更高的降解效率。在MB浓度5 mg/L、pH 6和OMPT浓度1.5 g /L的条件下,MB的降解率最快。总有机碳(TOC)分析表明,OMPT在240 min内实现了对MB的完全矿化,其速率常数高于P25和NPT的。
Abstract: Ordered mesoporous TiO2 (OMPT) was prepared by an evaporation induced self-assembly technique using liquid crystal as template. The key factors affecting the methylene blue (MB) oxidation efficiency were investigated, including the initial concentration of MB, pH value and catalyst concentration. The results show that the obtained OMPT has high thermal stability and shows a 2D hexagonal mesostructure with the small particle size and high surface area, which lead to higher degradation efficiency than commercial P25 or nanoparticle TiO2 (NPT) fabricated by sol-gel process. The optimal conditions are 5 mg/L MB, pH 6 and 1.5 g/L OMPT for the fastest rate of MB degradation. Total organic carbon (TOC) analysis indicates complete mineralization of MB in 240 min by OMPT, with rate constant higher than NPT or P25.
Trans. Nonferrous Met. Soc. China 24(2014) 1072-1078
Chen LIU, You-ji LI, Peng XU, Ze-shi LI, Meng-xiong ZENG
College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China
Received 20 May 2013; accepted 30 September 2013
Abstract: Ordered mesoporous TiO2(OMPT) was prepared by an evaporation induced self-assembly technique using liquid crystal as template. The key factors affecting the methylene blue (MB) oxidation efficiency were investigated, including the initial concentration of MB, pH value and catalyst concentration. The results show that the obtained OMPT has high thermal stability and shows a 2D hexagonal mesostructure with the small particle size and high surface area, which lead to higher degradation efficiency than commercial P25 or nanoparticle TiO2 (NPT) fabricated by sol-gel process. The optimal conditions are 5 mg/L MB, pH 6 and 1.5 g/L OMPT for the fastest rate of MB degradation. Total organic carbon (TOC) analysis indicates complete mineralization of MB in 240 min by OMPT, with rate constant higher than NPT or P25.
Key words: titania; ordered mesopore; liquid crystal template; methylene blue; degradation
1 Introduction
Industrialization and agricultural development, together with population growth, have drastically reduced clean water resources with various kinds of contaminants entering water. Developments in the field of chemical water purification have led to an improvement in oxidative degradation processes [1,2]. Recently, environmental purification using TiO2 as a photocatalyst has attracted a great deal of attention because of its chemical stability, robustness against photocorrosion, low toxicity, low pollution load, and availability at low cost [3,4]. However, the photoactivity of TiO2 should be improved in practical use for rapid degradation of organic contaminants. The photocatalytic efficiency of TiO2 is greatly influenced by crystal structure, particle size, surface area and porosity [5,6]. In particular, mesoporous nanocrystalline TiO2 has been demonstrated to be a more effective photocatalyst, because of its environmental friendliness, large surface area, ordered porous structure and large pore volume, which results in increasing surface reactive sites and improving mass transport [7,8]. It has been shown that hydrogen evolution is increased by a factor of up to 10 when ordered mesoporous TiO2 (OMPT) is applied instead of the bulk material [9]. It can thus be concluded that the introduction of porosity into these materials can be crucial to enhance their performance [10]. Ever since the first discovery of mesoporous TiO2 by ANTONELLI and YING [11] via a modified sol-gel method, a great deal of efforts have been concentrated on developing diverse techniques to synthesize ordered mesoporous TiO2. At first, only small-pore ordered mesoporous TiO2 could be obtained. CHOI et al [12] reported 2D hexagonal mesoporous TiO2 with the pore size of 7.3 nm. TSUNG et al [13] prepared mesoporous TiO2 submicrospheres with a mean pore size of 4 nm, combining the acetic acid mediated sol-gel chemistry system. Although mesoporous TiO2 materials have been widely used in the potential application fields of catalysis due to their large surface area, ordered pore networks, wide bandgap and small particle size, mesoporous structures would collapse after being heated at temperatures higher than 500 °C, in most cases due to the intrinsic crystallization of anatase phase. Up to now, the lower thermal stability is still a bottleneck for the practical applications of mesoporous TiO2. In an attempt to improve the thermal stability of the mesoporous TiO2 framework, some attempts to increase the thermal stability of mesoporous titania have intensely been explored by the special post-treatment and formation of thick pore walls [14,15]. Despite all these efforts, the fabrication of mesoporous titania with fully crystalline frameworks is still a great challenge, mainly because of the unmatching symmetries and high distortion energy between the mesostructure and crystallized TiO2.
CHMELKA et al [16] synthesized a cubic mesoporous TiO2 material with anatase in the walls, which was stable up to 400 °C. Since then, several post-synthesis thermal treatment strategies have been developed for the fabrication of thermally stable mesoporous TiO2 materials. Recently, our group has synthesized stable mesoporous TiO2 nanoparticles with high crystallinity, large surface area and small particle size via a post-treatment of the Soxhlet extraction, thermal stability over 500 °C. In the present work, an evaporation induced self-assembly (EISA) technique is applied to synthesizing ordered mesoporous titania nanomaterials with high surface area and thermal stability with liquid crystal as template. Meanwhile, the effects of photocatalytic conditions on OMPT photoactivity are studied, including MB concentration, OMPT concentration and pH value.
2 Experimental
2.1 Preparation of samples
The ordered mesoporous titania (OMPT) was prepared by an evaporation induced self-assembly (EISA) technique. Firstly, tetrabutyl orthotitanate (Aldrich, 99.9%; 25 mL) was dissolved in 50 mL of ethanol solution to synthesize titania sol with the controlling of hydrolysis rate by HCl. Secondly, accurately weighed CTAB (5 g) was completely dissolved in a certain amount of distilled water to form a hexagonal liquid crystal template (LCT). Then, the Ti sol was slowly dropped in the LCT with stirring. The obtained sample was evaporated at 80 °C for 4 h to exclude the ethanol in an oven. The final products were calcinated at 500 °C for 0.5 h after organics extraction with a Soxhlet apparatus during 48 h. The synthesis of OMPT was preformed by ELSA using LCT. In addition, TiO2 (NPT) nanoparticles were obtained as a reference using the same hydrolysis procedure for tetrabutyl orthotitanate without LCT by sol-gel method.
2.2 Characterization techniques
The samples obtained were characterized by measuring BET surface area by nitrogen absorption method (ASAP2010 of Micromeritics Company, USA) at 77 K. Single-point surface area was determined at p/p0=0.2. The small-angle X-ray scattering (SAXS) measurements were taken on a Nanostar U small-angle X-ray scattering system (Bruker, Germany) using Cu Kα radiation (40 kV, 35 mA). Wide-angle XRD (WAXRD) patterns were recorded on a Bruker D4 X-ray diffractometer with Ni-filtered Cu Kα radiation (40 kV, 40 mA). The morphology and porous structure of TiO2 in the prepared samples were observed by TEM (FEI Tecnai G20, US).
2.3 Photocatalytic activity evaluation
Methylene blue (MB) was chosen as a model organic compound to evaluate the photoactivity of the prepared samples and key factors affecting degradation. The particular photocatalytic course and setup were the same as the previously described [14]. The MB concentration was calculated from the absorbance at 660 nm using a calibration curve by a UV–Vis spectrometer (JascoV-500, Japan). The extent of mineralization was determined using a total organic carbon (TOC) analyzer (Euroglas TOC 1200).
3 Results and discussion
3.1 Characterization of prepared samples
Figure 1 shows the adsorption/desorption isotherm and the corresponding pore size distribution of the TiO2 microspheric sample after calcination at 500 °C. According to BDDT classification [17], the isotherm of OMPT sample in Fig. 1(a) exhibits type IV with a hysteresis loop, indicating the presence of mesopores, which means high surface area (127.3 m2/g). Moreover, OMPT has pronounced mesoporosity, narrow pore size distribution with pore radius in the range of 2 to 20 nm calculated using the BJH equation from the adsorption branch of the isotherm in Fig. 1(b). However, NPT shows low surface area (56.2 m2/g) with macropores in comparison with OMPT. The morphology and mesoporous structure of the obtained samples were further confirmed by TEM observations in Fig. 2. TEM image of OMPT sample shows a 2D hexagonal mesostructure in Fig. 2(a), which possesses highly ordered mesopores of about 4 nm in diameter. It also shows a high degree of periodicity of 2D hexagonal arrangements over large domains. From the HRTEM image (Fig. 2(b)), it can be seen that the lattice fringe corresponding to (101) (d101=0.35 nm) crystallographic planes of anatase indicates the high crystallinity of the pore walls of OMPT. However, from TEM image (Fig. 2(c)), there are no any mesoporous structures of NPT sample. The insets of Figs. 2(a) and (c) show the selected area electron diffraction (SAED) patterns for OMPT and NPT respectively, which present well resolved diffraction rings and many diffraction spots, indicating the high crystallinity of phase-pure anatase. The typical SAXS patterns of OMPT and NPT calcinated at 500 °C are shown in Fig. 3. There are two-resolved peaks which could be indexed as the (100) and (101) reflections observed on OMPT, suggesting a highly ordered 2D hexagonal structure. These results suggest that the obtained materials possess a highly organized mesoporous structure in comparison with NPT. The inset of Fig. 3 shows the WAXRD patterns of OMPT and NPT with the presence of nanocrystalline anatase because three high-intensity crystal peaks at 25.2°-55.1° could be observed and indexed as (110), (004) and (200), respectively (JCPDS, No. 21-1272) [15]. By calculation using Scherrer equation, the crystalline sizes of OMPT and NPT are 13.7 nm and 32.0 nm, respectively.
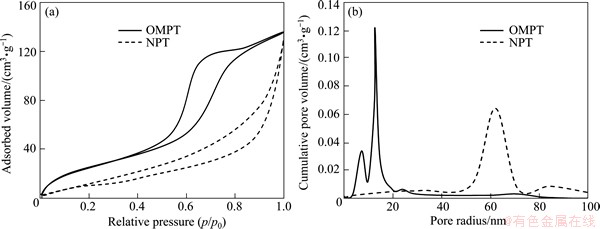
Fig. 1 N2 adsorption/desorption isotherm (a) and corresponding pore size distribution (b) of samples
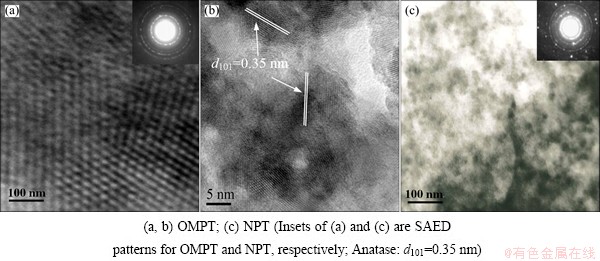
Fig. 2 TEM (a, c) and HRTEM (b) images of samples calcinated at 500 °C
3.2 Photocatalytic activity of samples
Under identical experimental conditions, it was found that only 2.1% and 2.7% of MB in solution were
absorbed on P25 and NPT in the dark after 1 h, respectively, while the amount of MB removed by the OMPT was 29.5%. This suggests that the greater adsorption of MB on OMPT compared with NPT is attributed to the higher surface area of OMPT (BET surface area of the catalysts: OMPT, 127.3 m2/g; NPT, 56.2 m2/g). The results of MB removal by the photocatalysts are presented in Fig. 4(a). The commercial Degussa P25 achieves only 80% MB removal in 160 min; however, OMPT achieves almost 100 % MB removal in the same time period. The photoactivity of TiO2 has been enhanced by mesoporous structure due to a synergetic effect of adsorption and photocatalytic decomposition of MB. To demonstrate the effect of mesoporous structure on photoactivity, photodecomposition of MB was studied using NPT without mesoporous structure. As shown in Fig. 4(a), the NPT has low decomposition rate of MB under UV irradiation in comparison with OMPT. It is regarded that the decomposition of MB mainly occurs on TiO2 particles and must be related to the structure of materials. The higher photoactivity of the OMPT than the NPT is related to two factors: a smaller particle size and a higher adsorptivity toward the organic substrate due to its high surface area, both of which could be cooperative in making the photocatalytic reaction of organic molecules more accessible to the active sites on the TiO2 surface. In Fig. 4(b), MB photocatalytic degradation is a pseudo-first-order reaction by P25 or NPT as well as OMPT due to high R2 value range from 0.96 to 0.99.
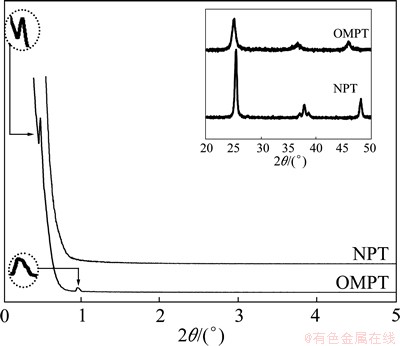
Fig. 3 SAXS and WAXRD patterns of samples calcinated at 500 °C for 0.5 h
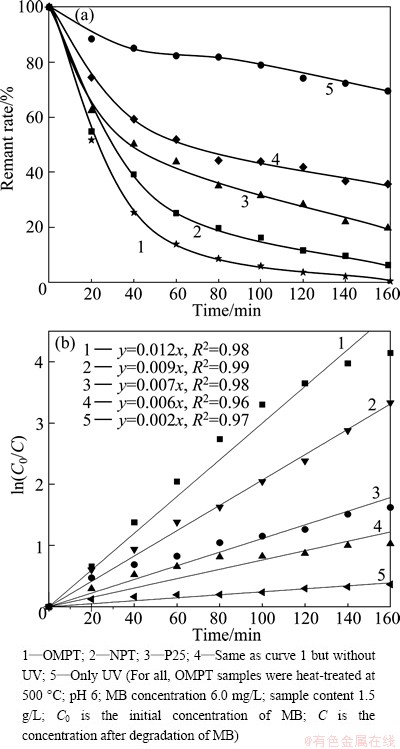
Fig. 4 Photocatalytic degradations for MB solution by samples
Temporal changes in the concentration of MB were detected by examining the variations in the maximal absorption UV–visible spectra at 660 or 665 nm [18]. Typically, MB solutions display the maximal absorbance at 668 and 609 nm [19]. Under our experimental conditions, the maximum absorption also occurred at 664 nm (Fig. 5). The band at 664 nm shifted considerably towards the blue region during the course of the photoassisted degradation. Related to the present observations, blue shifts of the absorption bands during the photooxidative N-deethylation of MB substrate in aqueous semiconductor dispersions were reported earlier by MOHAMMAD and MORRISON [17]. N-dealkylation of dyes containing auxochromic alkylamine groups plays an important role in photocatalytic degradation. The color of MB solutions becomes less intense (hypsochromic effect) when all or part of the auxochromic groups (methyl or methylamine) degrade. Figure 5 also shows that the spectral band at 664 nm blue-shifts by as much as 6 nm from 664 to 670 nm during the course of the photodegradation. It suggests that MB is N-demethylated in a stepwise manner (i.e., methyl groups are removed one at a time as confirmed by the gradual peak wavelength shifts toward the blue region), with cleavage of the MB chromophore ring structure (phenothiazine or thionine) occurring concomitantly as evidenced by the decrease in TOC (see below). Absorption bands of N-demethylated analogs of MB in the visible range are from 655 to 603 nm [20]. As weak electron-donor substituents, methyl groups can facilitate attack on MB by electrophilic species (·OH or 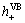 ) in the demethylation process; this is also likely to be a major step in the photocatalytic oxidative degradation of MB. And then mixtures of colored intermediates complete N-demethylation, deamination and entirely degradation during irradiation, which is consistent with the significant decrease of total bands and appearance of no new bands.
) in the demethylation process; this is also likely to be a major step in the photocatalytic oxidative degradation of MB. And then mixtures of colored intermediates complete N-demethylation, deamination and entirely degradation during irradiation, which is consistent with the significant decrease of total bands and appearance of no new bands.
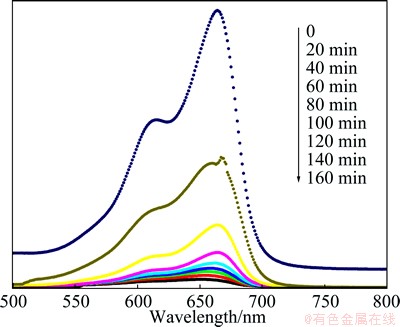
Fig. 5 Temporal spectral changes of MB in aqueous OMPT suspensions under UV illumination
Fig. 6 Effect of initial MB concentration (a), OMPT concentration (b) and pH (c) on degradation rate of MB solution
3.3 Effect of photocatalytic conditions on degradation efficiency of MB
The effect of initial MB concentration on degradation rate was studied by varying the initial concentration from 5 to 9 mg/L, and the results are depicted in Fig. 6(a). It is clear that the degradation rate kapp is from 12.2×10–3 to 2.0×10–3 min–1 in the MB concentration range of 5-9 mg/L. The results show that the kapp decreases with an increase in the initial concentration of MB. This can be a result of blocking of the photocatalytically active sites on the surface of catalyst particles by MB molecules and reducing the interaction of photos with these sites. Another possible reason is that a mass of UV light may be absorbed by the MB molecules in aqueous solution rather than the catalyst particles for high MB concentration, which can also reduce UV light adsorption of catalysts to a certain extent [19]. Moreover, the increase of initial concentration of MB provides further negative effects on its degradation, leading to the efficiency of the catalytic reaction. The reaction rate as a function of catalyst concentration is important [21]. Hence a series of experiments were carried out to find the optimum catalyst concentration by varying OMPT concentration from 1.3 to 2.1 g/L (Fig. 6(b)). It is observed that there is a steady increase in the degradation efficiency up to 1.5 g/L of the catalyst beyond which the degradation efficiency decreases. This can be explained as follows. The turbidity of the solution above 1.5 g/L reduces the light transmission through the solution, while below this level the adsorption on TiO2 surface and the absorption of light by TiO2 are the limiting factors. WEI and WAN [22] also reported that the catalyst concentration has both positive and negative impacts on the photodecomposition rate. The increased concentration of catalyst increases the quantity of photons absorbed and consequently the degradation rate. Further increase in the catalyst concentration beyond 1.5 g/L may result in the deactivation of activated molecules due to collision with the ground state molecules [23]. At concentrations higher than 1.5 g/L, OMPT aggregation (particle–particle interactions) may commence and lower the effective surface area of the catalyst, and adsorption of the reactant.
The amphoteric behaviour of titania influences the surface charge of the photocatalyst. The role of pH in the degradation rate was studied in the pH range of 4-8. The results are shown in Fig. 6(c). It is observed that the rate of degradation increases with an increase in pH, exhibiting a maximum at pH 6, and then decreases. Strong acid or alkali is not available for decomposing MB due to the fact that the amount of hydroxy absorption on TiO2 is influenced by pH in solution [24]. The low or high pH value is not available for MB absorption and hydroxy produce on OMPT. So, there is an optimum pH in the photocatalytic process of MB because high concentration hydroxy and plentiful MB which are absorbed on OMPT are available for the photocatalytic reaction.
3.4 Mineralization studies
The extent of mineralization of MB was followed by total organic carbon (TOC) analysis as shown in Fig. 7. As irradiation time increases, MB molecules degrade into fragments and consequently mineralize completely. The decrease in degradation rate after about 2 h is due to the formation of less polar intermediates and their poor adsorption on the surface of titania. The TOC results show that P25 and NPT require 320 min and 280 min for complete mineralization, respectively, whereas OMPT requires only 240 min.
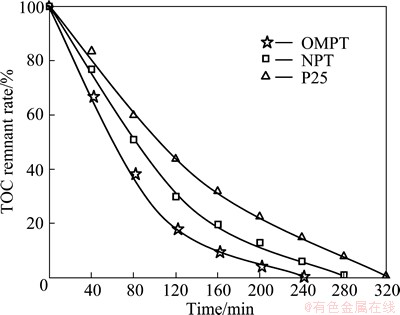
Fig. 7 TOC remnant rate of MB in photocatalytic degradation process
4 Conclusions
1) OMPT calcined at 500 °C has a high thermal stability and shows 2D hexagonal mesostructure with the particle size in the range of 20-30 nm, crystallite size of 9.4 nm, specific surface area of 127.3 m2/g, and narrow pore size distribution with pore radius in the range of 2-20 nm.
2) OMPT shows a higher photocatalytic efficiency for MB degradation than NPT or commercial Degussa P25.
3) The initial concentration of MB influences the photoactivity of OMPT. For a MB concentration of 5 mg/L, OMPT showed the highest decomposition velocity. In addition, the pH value and catalysts concentration also influenced the photoactivity of OMPT. pH 6 and 1.5 g/L of OMPT concentration were suitable for MB degradation.
References
[1] VAMATHEVAN V, AMAL R D, BEYDOUN G L. Photocatalytic oxidation of organics in water using pure and silver-modified titanium dioxide particles [J]. Photochem Photobiol A, 2002, 148: 237-245.
[2] TAO J, DENG J, DONG X, ZHU H, TAO H J. Enhanced photocatalytic properties of hierarchical nanostructured TiO2 spheres synthesized with titanium powders [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2049-2056.
[3] TAO T, CHEN Q Y, HU H P, YIN Z L, CHEN Y. TiO2 nanoparticles prepared by hydrochloric acid leaching of mechanically activated and carbothermic reduced ilmenite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1232-1238.
[4] LI Y J, LI X D, LI J W, YIN J. Photocatalytic degradation of methyl orange in a sparged tube reactor with TiO2-coated activated carbon composites [J]. Catal Commun, 2005, 6: 651-655.
[5] MINERO C, CATOZZO F, PELIZZETTI E. Role of adsorption in photocatalyzed reactions of organic molecules in aqueous TiO2 suspensions [J]. Langmuir, 1992, 8: 481-486.
[6] MATOS J, LAINE J, HERRMANN J M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon [J]. Appl Catal B, 1998, 18: 281-291.
[7] TANG J, WU Y, MCFARLAND E W, STUCKY G D. Synthesis and photocatalytic properties of highly crystalline and ordered mesoporous TiO2 thin films [J]. Chem Commun, 2004, 14: 1670-1671.
[8] PARK S E, KIM B E, LEE S W, LEE J K. Employment of encapsulated Si with mesoporous TiO2 layer as anode material for lithium secondary batteries [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 1023-1026.
[9] CREPALDI E L, SOLERIIA G J, GROSSO D, CAGNOL F, RIBOT F, SANCHEZ C. Controlled formation of highly organized mesoporous titania thin films: From mesostructured hybrids to mesoporous nanoanatase TiO2 [J]. J Am Chem Soc, 2003, 125: 9770-9786.
[10] WU L, YU J C, WANG X C, ZHANG L Z, YU J G. Characterization of mesoporous nanocrystalline TiO2 photocatalysts synthesized via a sol-solvothermal process at a low temperature [J]. J Solid State Chem, 2005, 178: 321-328.
[11] Antonelli D M, Ying J Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method [J]. Angew Chem Int Ed, 1995, 34: 2014-2017.
[12] CHOI S, MAMAK M, COOMBS N, OZIN G. Thermally stable two-dimensional hexagonal mesoporous nanocrystalline anatase, meso-nc-TiO2: Bulk and crack-free thin film morphologies [J]. Adv Funct Mater, 2004, 14: 328-335.
[13] Tsung C K, Fan J, Zheng N, Shi Q, Forman A J, Wang J, Stucky G D. A general route to diverse mesoporous metal oxide submicrospheres with highly crystalline frameworks [J]. Angew Chem Int Ed, 2008, 47: 8682-8686.
[14] ZHANG Z, ZUO F, FENG P. Hard template synthesis of crystalline mesoporous anatase TiO2 for photocatalytic hydrogen evolution [J]. Mater Chem, 2010, 20: 2206-2212.
[15] ZHOU W, SUN F F, PAN K, TIAN G H, JIANG B J, REN Z Y, TIAN C G, FU H G. Well-ordered large-pore mesoporous anatase TiO2 with remarkably high thermal stability and improved crystallinity: preparation, characterization, and photocatalytic performance [J]. Adv Funct Mater, 2011, 21: 1922-1930.
[16] CHMELKA B F, STUCKY G D, ALBERIUS P C A, FRINDELL K L, HAYWARD R C, KRAMER E J.General predictive syntheses of cubic, hexagonal, and lamellar silica and titania mesostructured thin films [J]. Chem Mater, 2002, 14(8): 3284-3294.
[17] LAKSHMI S, RENGANATHAN R, FUJITA S. Study on TiO2-mediated photocatalytic degradation of methylene blue [J]. Photochem Photobiol A, 1995, 88: 163-167.
[18] MOHAMMAD T, MORRISON H. Simultaneous photoconjugation of methylene blue and cis-rh(phen)2Cl2+ to DNA via a synergistic effect [J]. Photochem Photobiol A, 2000, 71: 369-381.
[19] TALEBIAN N, NILFOROUSHAN M R. Comparative study of the structural, optical and photocatalytic properties of semiconductor metal oxides toward degradation of methylene blue [J]. Thin Solid Films, 2010, 518(8): 2210-2215.
[20] ZHANG T Y, Oyama T, Aoshima A, Hidaka H, Zhao J, Serpone N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation [J]. Photochem Photobiol, 2001, 140: 163-172.
[21] KUO W S, HO P H. Solar photocatalytic decolorization of methylene blue in water [J]. Chemosphere, 2001, 45(1): 77-83.
[22] WEI T Y, WAN C C. Heterogeneous photocatalytic oxidation of phenol with titanium dioxide powders [J]. Ind Eng Chem Res, 1991, 130: 1293-1300.
[23] CHEN L C, CHOU T C. Photobleaching of methyl orange in titanium dioxide suspended in aqueous solution [J]. Mol Catal, 1993, 85: 201-208.
[24] RIZZO L. KOCH J, BELGIORNO V, ANDERSON M A. Removal of methylene blue in a photocatalytic reactor using polymethylmethacrylate supported TiO2 nanofilm [J]. Desalination, 2007, 211(1-3): 1-9.
刘 晨,李佑稷,徐 鹏,李泽时,曾孟雄
吉首大学 化学化工学院,吉首 416000
摘 要:利用蒸发诱导自组装技术,用液晶为模板制备有序介孔氧化钛(OMPT),探讨影响亚甲基蓝(MB)氧化降解效率的主要因素,包括MB的初始浓度、pH值和催化剂浓度。结果表明,所获得的OMPT具有二维六方介孔结构,粒径小,比表面积大,表现出高的热稳定性,这些都导致其比催化剂P25和溶胶–凝胶法制备的纳米氧化钛颗粒(NPT)有更高的降解效率。在MB浓度5 mg/L、pH 6和OMPT浓度1.5 g /L的条件下,MB的降解率最快。总有机碳(TOC)分析表明,OMPT在240 min内实现了对MB的完全矿化,其速率常数高于P25和NPT的。
关键词:二氧化钛;有序介孔;液晶模板;亚甲基蓝;降解
(Edited by Hua YANG)
Foundation item: Project (51172092) supported by the National Natural Science Foundation of China; Project (11A093) supported the Education Department of Hunan Province, China; Project (13JJ1023) supported by the Natural Science Fund for Distinguished Youth of Hunan Province, China; Project (NECT-12-0720) supported the Program for New Century Excellent Talents in Universities of China
Corresponding author: You-ji LI; Tel: +86-13762157748; E-mail: bcclyj@163.com
DOI: 10.1016/S1003-6326(14)63164-2

