
Surface charge properties of red mud particles generated from
Chinese diaspore bauxite
ZHANG Kun-yu(张琨瑜), HU Hui-ping(胡慧萍), ZHANG Li-juan(张丽娟), CHEN Qi-yuan(陈启元)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 24 September 2007; accepted 21 March 2008
Abstract: Acid/basic potentiometric titration can be used to quantify the red mud surface charge properties. The amount of surface active —OH groups and surface charge density on the red mud particles generated from Chinese diaspore bauxite were evaluated from the acid/basic potentiometric titration data in 0.1 mol/L or 0.5 mol/L NaCl solution. The results show that the adsorption of sodium polyacrylate(SPA) on the red mud surface causes the increase of the surface active —OH groups, which makes the point of zero charge(PZC) shift to a lower pH value. However, the adsorption of polyacrylamide(PAM) causes little change. As the concentration of NaCl solution increases, the surface charge becomes more positive in acidic solution and more negative in alkaline solution, which can be attributed to the presence of a porous surface gel coating on the red mud particles.
Key words: red mud; surface charge; point of zero charge; potentiometric titration; bauxite
1 Introduction
Red mud must be separated from aluminate solution after alumina digestion and the separation process is often enhanced by adding polymeric flocculants. The flocculation effectiveness is determined by the flocculation property of polymeric flocculants and red mud surface property[1]. Red mud contains oxides of iron, aluminium, silicon and titanium, which makes red mud surface bear lots of hydroxyl groups. The groups responsible for the red mud surface charge properties are important for the interaction between red mud and flocculant molecules serving as active sites[2].
The amount of red mud surface active —OH groups and the red mud surface charge density can be determined by means of acid/basic potentiometric titration. This method has been widely used to characterize the surface hydroxyl groups of oxides and clay minerals[3-4], and was used to determine the surface charge properties of activated or pretreated red mud particles[5-6]. CHVEDOV et al[2] studied the surface charge properties of red mud particles generated from different kinds of bauxites such as Jamaican gibbsite bauxite and Australian boehmite bauxite, and determined the amount of surface active —OH groups and the PZC value of red mud particles flocculated with high molecular mass sodium polyacrylate by employing the acid/basic potentiometric titration. In this study, an attempt was made to evaluate the surface charge properties of some focculated and unflocculated red mud particles generated from Chinese diaspore bauxite in NaCl solution using the acid/basic potentiometric titration.
2 Experimental
2.1 Materials
Bayer red mud slurry generated from Chinese diaspore bauxite was obtained from a bauxite refinery of China Aluminium Co. Ltd (CHALCO). The red mud slurry was split into three parts, with two parts being flocculated by sodium polyacrylate(SPA) and polyacrylamide(PAM), respectively. Each part was then separated by centrifugation and the residual alkali lye in the centrifuge cake of red mud was removed first by hot water washing. Subsequently, the red mud was cleaned with dilute HCl solution and then distilled water until there was a constant pH value in red mud suspension. Finally, the thoroughly cleaned red mud was dried at 105 ℃ for 2 h. The red mud had an average grain size of about 10 μm measured by a Mastersize 2000 laser granulometer (Malvern, U.K.) and the chemical compositions are listed in Table 1. The BET method with N2 adsorption on a Micromeritics ASAP2020 auto- adsorption analyzer (MIKE, U.S.A.) was used to obtain the red mud surface properties. The results are listed in Table 2.
Table1 Chemical compositions of dry red mud (mass fraction, %)

Table 2 Some surface properties of dry red mud

SPA (Tianjin Kermel Development Centre of Chemical Reagents, China) and PAM (Agents for Chemicals in Shanghai, China) were commercially pure and used to flocculate red mud. The average molecular mass of SPA and PAM were 8-10 million and about 3 million, respectively. The dosage of flocculants was 200 g per ton dry red mud.
2.2 Acid/basic potentiometric titration
The surface charge of red mud particles was determined using the acid/basic potentiometric titration technique described in Ref.[7]. About 0.13 g dry red mud was placed into stopper-bearing glass bottles with 70 mL of 0.1 mol/L or 0.5 mol/L NaCl solution. The red mud suspension was then equilibrated for approximately 14 h on a reciprocal shaker, then the pH value of the suspension was determined by a digital pH-meter (model PHS-10B, China). The titrations began at pH 4 and ended at pH 11 to avoid red mud dissolution. Two identical suspensions were completely titrated from pH 4 to 11, separately, with 1 mol/L HCl and with 1 mol/L NaOH by using a microburette. After each HCl or NaOH addition, the suspension was shaken for 14 h, then the pH value was recorded. The temperature of the whole titration process maintained at 25 ℃.
3 Results and discussion
3.1 Total amount of surface active —OH groups on red mud particles
Titration curves exhibit the response of the red mud system to the changes in the pH value as acid or base is added. The typical potentiometric titration curves of the slurries in 0.1 mol/L or 0.5 mol/L NaCl solution are shown in Figs.1-3.
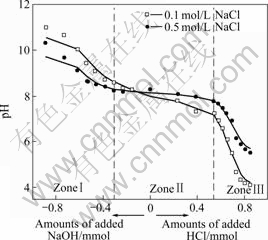
Fig.1 Titration curves of unflocculated red mud slurry
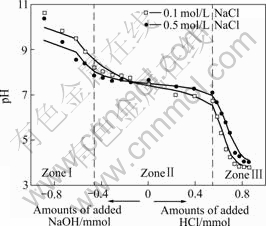
Fig.2 Titration curves of red mud slurry flocculated with SPA
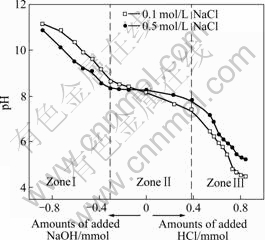
Fig.3 Titration curves of red mud slurry flocculated with PAM
by such atoms as Fe, Al and Ti since the oxides of these elements are the main compositions of the red mud.
The red mud surface active —OH groups (represented by Si—OH) are able to adsorb or desorb H+. Si—OH comes from the surface structural hydroxyl groups of red mud or the carboxyls of SPA adsorbed on red mud surface. The general acid-base equilibrium can be written in Eqn.(1), where the Si atom can be replaced

(1)
As seen in Figs.1-3, the addition of the red mud particles to NaCl solution results in inflection in titration curves, each of which is split into three zones (ZonesⅠ-Ⅲ). In basic aqueous solution (ZoneⅠ), the slurry particles carry ionized surface active —OH groups (Si-O–) with exposed oxygen atoms on the red mud surface and can consume protons. In this stage, the pH drop with HCl addition is only due to the consumption of protons to neutralize free OH– ions in NaCl solution. Because free OH– ions are more mobile and accessible, they are first consumed in titration. Near the neutral pH value, almost all free OH– ions are consumed during titration and there is a sharp drop of the pH value to another stage (Zone Ⅱ). Titration curves are flatten out to some extent depending on the red mud type to form Zone Ⅱ, where only surface active —OH groups (Si—O– or Si—OH) form Si—OH or Si—OH2+. Because all the added HCl is consumed by the surface active —OH groups in titration, a constant pH value is present. The first bend between ZoneⅠand Zone Ⅱ, and the second bend between Zone Ⅱand Zone Ⅲ in titration curves respectively correspond to the beginning and the end of the titration. Therefore, the total amount of the surface active —OH groups of the red mud can be calculated from Zone Ⅱ of the curves by Eqn.(2) or Eqn.(3) and the results are shown in Table 3. In Zone Ⅲ of Figs.1-3, the drop in the pH values is due to the accumulation of protons as HCl is added.

where N1Si—OH and N2Si—OH are the total amount of the red mud surface active -OH groups in mol/kg and mol/m2, respectively, ΔnⅡ is the mole amount of HCl consumed in Zone Ⅱ, mRM is the mass of the dry red mud in the suspension in kg, ARM is the total red mud surface area in m2, and “2” indicates that the red mud particles can consume twice protons as many as the total red mud surface active -OH groups.
Table 3 Total amounts of red mud surface active —OH groups
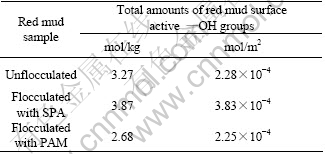
As indicated in Table 3, the coating of SPA on the red mud surface results in an increase in the total amount of surface active —OH groups. And the coating of PAM causes little change in the total amount of —OH groups in the unit of mol/m2, but an obvious decrease in the unit of mol/kg. During flocculation in caustic solution, the —COONa groups in the polymeric chain of SPA are ionized to —COO- groups, which combine with hydroxyls of the red mud surface by hydrogen bond and are consumed[8]. However, there are also lots of free —COO- groups on the mud surface against this consump- tion. As a result, the total amount of the active —OH groups on the mud surface increases with SPA coating on the surface, which accords with the results by CHVEDOV et al[2], where the surface active —OH groups of Bayer red mud from Jamaican gibbsite bauxite, and from Australian boehmite bauxite are increased by flocculation, respectively, from 1.2×10-4 mol/m2 to 2.7×10-4 mol/m2 and from 11.9×10-4 mol/m2 to 12.4×10-4 mol/m2.
Red mud separation in alumina production is operated in the basic condition, which can cause the hydrolysis of some —CONH2 to form —COO- in PAM polymer chains[9]. Then partially hydrolyzed PAM adsorbs on the red mud surface via hydrogen bond, which reduces the surface active hydroxyl groups. The amounts of free carboxyls from partially hydrolyzed PAM and their —OH groups are close to the decreased amounts of the hydroxyls on the mud surface due to PAM adsorption. Thus, the total amount of active —OH groups per unit area on the mud surface almost doesn’t change. But the red mud surface active —OH groups per unit mass decrease obviously, due to the aggregation of flocs and the reduction of specific surface area of the mud after the flocculation with PAM (as shown in Table 2).
3.2 Surface charge density of red mud particles
The surface charge of red mud particles can be derived from acid/basic titration because it is usually governed by the proton’s adsorption/desorption of the surface active —OH groups. The surface charge density (σ) can be calculated from the titration curves (Figs.1-3) and Eqn.(4)[10]:
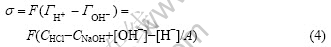
where σ is the surface charge density (C/cm2), F is the Faraday constant, 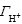 and
and 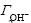 are the adsorbed amounts of H+ and OH- (mol/cm2), respectively, CHCl and CNaOH are the concentrations of the added HCl and NaOH in NaCl solution (mol/cm3), respectively, [H+] and [OH-] are the concentrations of H+ and OH- in NaCl solution obtained from pH measurements (mol/cm3), and A is the total red mud surface area per unit volume of NaCl solution (cm2/cm3).
are the adsorbed amounts of H+ and OH- (mol/cm2), respectively, CHCl and CNaOH are the concentrations of the added HCl and NaOH in NaCl solution (mol/cm3), respectively, [H+] and [OH-] are the concentrations of H+ and OH- in NaCl solution obtained from pH measurements (mol/cm3), and A is the total red mud surface area per unit volume of NaCl solution (cm2/cm3).
The curves of the surface charge density (σ) vs the pH value for the red mud are presented in Figs.4-6, respectively. The results in Figs.4-6 demonstrate that the overall pH dependence of the surface charge density (σ) has a similar shape for either flocculated or unflocculated red mud. The surface charge properties can be represented by the point of zero charge (PZC) defined as the pH value at which the net charge on the surface is zero. The PZC value of the red mud is located at the intersection[5-11] of acid/basic potentiometric titration curves in Figs.4-6 and listed in Table 4.
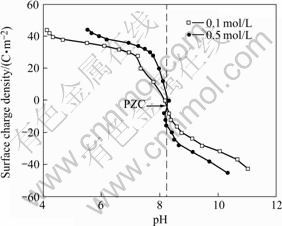
Fig.4 Surface charge of unflocculated red mud slurry
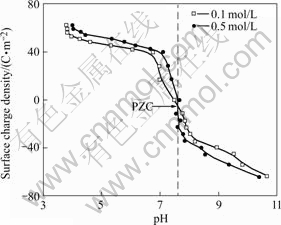
Fig.5 Surface charge of red mud slurry flocculated with SPA
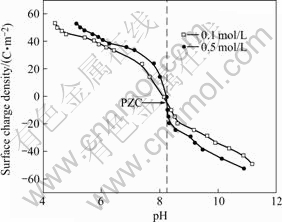
Fig.6 Surface charge of red mud slurry flocculated with PAM
Table 4 PZC of red mud

As seen in Table 4, the PZC value is minimal for the red mud by flocculation with SPA. The adsorption of SPA with —COO- groups on the surface makes the negative surface charge increase. Neutralizing the negative charge calls for more H+, resulting in the decrease in PZC. The PZC value of the red mud flocculated with PAM is almost equal to that of the unflocculated red mud, because the adsorption of PAM causes little change in the amount of the —OH groups of surface.
In the previous study[12], the PZC value of the unflocculated red mud from gibbsite bauxite is 8.1±0.2, which equals to 8.25 of the present study within the errors. The red mud is a complex of such oxides as Fe2O3, Al2O3, SiO2 and TiO2, and its PZC value lies on the contents and the PZC values of these oxides[13], which are 8.5, 9, 2, and 7, respectively[11]. Here only SiO2 has an acidic PZC value. The red mud containing more silica should have a lower PZC value. The content of SiO2 of the present red mud is limited (Table 1), therefore there is an alkaline PZC. Chinese diaspore has much worse digestion performance than gibbsite, e.g. the digestion process usually calls for lots of CaO added to improve the leaching rate of Al2O3. As a result, Bayer red mud from this process contains a great amount of CaO, while the red mud from gibbsite bauxite contains over 37% Fe2O3[12], which is much higher than that of diaspore used in the present study. Therefore, the PZC values of the red mud both from gibbsite and diaspore are almost equal because the PZC value of CaO is 8.1, very close to the PZC value of Fe2O3[14].
Figs.4-6 demonstrate that the red mud surface in equilibrium with H+ or OH- ions has a zero charge at the PZC value by the formation of neutral Si—OH. The positive charge of the red mud at pH below the PZC value is attributed to chemisorption of H+ forming Si—OH2+ on the neutral surface. And at pH above the PZC value, the negative charge on the red mud surface forms Si—O–. In the region near PZC, the surface charge density (σ) drops sharply with the pH increasing, which is due to acid/basic titration of the red mud surface —OH groups. Under much more basic or acidic condition, the charge change is the result of partial dissolution of some components in the red mud.
As shown in Figs.4-6, the surface charges are extended at higher ionic strengths. This ion effect can be attributed to the presence of a porous surface gel coating on the red mud particles. The gel coating is helpful to ion transport and is formed during the shake equilibrium process of red mud suspension. Charged surface sites of the red mud do not directly contact with NaCl solution because of the porous gel layer. H+ ions may diffuse through the porous gel layer to the inner layer to change the charge density of the red mud surface[11]. In NaCl solution with the suspension mud, Na+ ions are adsorbed on the porous surface layer and play the role of transferring H+. As NaCl concentration increases, the amount of Na+ ions adsorbed on the porous surface layer increases, which can promote the diffusion of H+ ions from bulk solution to the mud surface to increase the positive surface charge under acidic condition, or the diffusion of H+ ions in surface active —OH groups to bulk solution to increase the negative surface charge under basic condition.
As shown above, the red mud particles carry the negative charge under a strongly basic condition. Digesting liquor containing the red mud in alumina industry usually has a pH above 12 and therefore it carries the negative surface charge. In general, the flocculants for the red mud separation are anionic. Since both the red mud and flocculants carry negative charge, flocculant molecules can adsorb on red mud surface only by nonelectrostatic attraction such as hydrogen bond, interaction via Na+ or Ca2+ bridge[15-16] and so on.
4 Conclusions
1) The acid/basic potentiometric titration was used to characterize the red mud surface charge properties. Due to —COO- groups in the polymeric chain of SPA, the adsorption of SPA on the red mud surface results in the increase of the slurry surface active —OH groups, but the adsorption of PAM causes little change.
2) The PZC value of the red mud relates to the type of flocculants. The SPA adsorption produces a shift in the PZC value toward a lower pH value, while the PZC value of the red mud flocculated with PAM is close to that of the unflocculated.
3) The red mud carries a positive charge in strongly acidic solution and a negative charge in strongly caustic solution. With the increases of the NaCl concentration, the surface charge becomes more positive in acidic region and more negative in alkaline region.
References
[1] HIND A R, BHARGAVA S K, GROCOTT S C. The surface chemistry of Bayer process solids: A review [J]. Colloids Surf A, 1999, 146(3): 359-374.
[2] CHVEDOV D, OSTAP S, LE T. Surface properties of red mud particles from potentiometric titration [J]. Colloids Surf A, 2001, 182(2): 131-141.
[3] FOKKINK L G J, DE KEIZER A, LYKLEMA J. Temperature dependence of the electrical double layer on oxides: Rutile and hematite [J]. J Colloid Interface Sci, 1989, 127(1): 116-131.
[4] RODRIGUES F A, MONTEIRO P J M, SPOSITO G. The effect of monovalent and bivalent cations on the surface charge of opal [J]. Cement and Concrete Research, 2001, 31(11): 1549-1552.
[5] APAK R, GUCLU K, TURGUT M H. Modeling of copper(Ⅱ) , cadmium(Ⅱ), and lead(Ⅱ) adsorption on red mud [J]. J Colloid Interface Sci, 1998, 203(1): 122-130.
[6] PRADHAN J, DAS S N, THAKUR R S. Adsorption of hexavalent chromium from aqueous solution by using activated red mud [J]. J Colloid Interface Sci, 1999, 217(1): 137-141.
[7] NIU Z X, CAO F H, WANG W, ZHANG Z, ZHANG J Q, CAO C N. Electrodeposition of Ni-SiC nanocomposite film [J]. Trans Nonferrous Met Soc China, 2007, 17: 9-15.
[8] NASSER M S, JAMES A E. The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions [J]. Separation and Purification Technology, 2006, 52: 241-252.
[9] FANG D B, GUO R W, HA R H. Acrylamide polymers [M]. Beijing: Chemical Industry Press, 2006. (in Chinese)
[10] RODRIGUES F A, MONTEIRO P J M, SPOSITO G. The surface charge density of silica and its effect on expansive pressure [J]. Cement and Concrete Research, 1999, 29(4): 527-530.
[11] ATUN G, HISARLI G. A study of surface properties of red mud by potentiometric method [J]. J Colloid Interface Sci, 2000, 228(1): 40-45.
[12] APAK R, TUTEM E, HUGUL M, HIZAL J. Heavy metal cation retention by unconventional sorbents (red muds and fly ashes) [J]. Wat Res, 1998, 32(2): 430-440.
[13] PARIDA K, SATAPATHY P K, DAS N. Studies on Indian ocean manganese nodules [J]. J Colloid Interface Sci, 1996, 181(2): 456- 462.
[14] HANAWA H, KON M, DOI H, UKAI H. Amount of hydroxyl radical on calcium-ion-implanted titanium and point of zero charge of constituent oxide of the surface-modified layer [J]. J Materials Sci, 1998, 9(1): 89-92.
[15] CHEN H T, RAVISHANKAR S A, FARINATO R S. Rational polymer design for solid–liquid separations in mineral processing applications [J]. Int J Miner Process, 2003, 72(1): 75-86.
[16] CARLOS N, LUIS M S, FUENTE E. Polyacrylamide induced flocculation of a cement suspension [J]. Chemical Engineering Science, 2006, 61: 2522-2532.
Foundation item: Project(2005CB623702) supported by the National Basic Research Program of China
Corresponding author: HU Hui-ping; Tel: +86-731-8877364; Fax: + 86-731-8879616; E-mail: phhuiping@hotmail.com
(Edited by YUAN Sai-qian)