J. Cent. South Univ. Technol. (2008) 15: 599-605
DOI: 10.1007/s11771-008-0112-x
Synthesis and fluorescence properties of Tb(Ⅲ) complexes with pyridine-2,6-dicarboxylic acid derivatives
TANG Rui-ren(唐瑞仁), ZHENG You-hu(郑由浒), GU Guo-liang(顾国梁)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: Two novel ligands named 4-styrylpyridine-2,6-dicarboxylic acid (4-SPDA) and 4-(4-(2-(2, 6-dicarboxypyridin-4-yl)- vinyl)styryl)pyridine-2,6-dicarboxylic acid(DSPDA) and their complexes with Tb(Ⅲ) were synthesized and characterized by infrared spectrometry, 1H nuclear magnetic resonance, elemental analysis and gas chromatograph-mass spectrometry. The ligand synthetic route was optimized. The fluorescence properties of the complex in solid state, in different kind of solvents and in solutions with different pH values were investigated in detail. The results show that the yields of DSPDA and 4-SPDA reach over 78% by Wittig-Horner reaction and other eight pyridine-2, 6-dicarboxylic acid derivatives with different substituents on pyridine ring, and their complexes with Tb(Ⅲ) are also obtained. The fluorescence intensities of the complexes with electron-donating groups are more intense than those of the complexes with electron-withdrawing groups on pyridine ring; fluorescence intensities of the complexes are the strongest in neutral solution (pH=7), and the less the dipole moment of solvent molecule is, the stronger the fluorescence intensity is. It is found that the two ligands (4-APDA and DSPDA) are the good sensitizers for Tb(Ⅲ) ion.
Key words: Tb(Ⅲ) complexes; pyridine-2, 6-dicarboxylic acid derivatives; synthesis; fluorescence property
1 Introduction
The inner-shell nature of 4f electrons results in lanthanide trivalent ions, Ln(Ⅲ), having remarkable spectroscopic properties, with narrow and easily recognizable f–f transitions; Ln(Ⅲ) chelates give narrow-band emissions and large Stokes displacement of the Ln(Ⅲ) ions under UV–Vis excitation. The exciting light energy absorbed by the organic chromophore is transferred to a triplet state of the ligand by the intersystem crossing, and then intra-molecularly transferred to the Ln(Ⅲ) ion. This sensitized luminescence of lanthanides is widely applied in many fields, such as probes and labels in a variety of chemical and biological applications[1-3], organic electro- luminescence devices(OELDs) that utilize several lanthanide complexes especially with Eu(Ⅲ) and Tb(Ⅲ) organic complexes as luminescent centers[4]. A lot of work has been done to design and synthesize lanthanide chelates with good luminescence properties[5]. Pyridine-2, 6-dicarboxylic acid(PDA) derivatives used as sensitizers are better than other ligands. Since lanthanide complexes with PDA derivatives are chiral, they can get more information about structure of biological molecule through measurement of circularly polarized luminescence spectra[6-7], and can be used to tag proteins in time-resolved fluoroimmunoassay (TR-FIA) successfully.
In this work, two novel ligands named 4-styrylpyridine-2,6-dicarboxylic acid (4-SPDA) and 4-(4-(2-(2,6-dicarboxypyridin-4-yl)vinyl)styryl)pyridine-2,6-dicarboxylic acid (DSPDA) and their complexes of Tb(Ⅲ) with rigid planar structure and excellent electronic negotiability in the conjugated plane [8] were designed and synthesized. The two pyridine rings of DSPDA can chelate with two lanthanide ions respectively to form a π-extended conjugated netted structure, which can increase the fluorescence efficiency. In addition, other eight Tb(Ⅲ) complexes of pyridine-2, 6-dicarboxylic acid derivatives with different substituents on pyridine ring were also prepared, and the fluorescence properties of Tb(Ⅲ) complexes in solid state, in different kind of solvents and in solutions with different pH value were studied.
2 Experimental
2.1 Reagents and instruments
Melting points of the synthesized compounds were determined on an XRC-1 apparatus. Infrared (IR) spectra were recorded on an Avatar-360-FT spectrophotometer with KBr plates. All nuclear magnetic resonance(NMR) spectra were recorded at 270 K with an INOVA-400 apparatus, and gas chromatograph-mass spectra (GC-MS) were performed on an HP6890-HP5973 gas chromatograph-mass united spectrometer. Fluorescence properties were recorded on an FL-2500 apparatus, elemental analysis(EA) of elements C, H and N was performed on a Perkin-Elmer 2400 elemental analyzer.
Pyridine-2,6-dicarboxylic acid was obtained from Jiuzhou Chemical Plant of China. 4-(hydroxylmethyl) pridine-2,6-dicarboxylate (Compound 1) and p-xylylene bis-(triphenylphosphonium bromide) (Compound 2) were prepared according to Refs.[9-10]. Others reagents were commercial analytical or chemical pure grades and were not purified additionally.
2.2 Preparation of ligands
4-hydroxypyridine-pyridine-2,6-dicarboxylic acid (HPDA), 4-methoxypyridine-2,6-dicarboxylic acid (MPDA), 4-aceta-midopyridine-pyridine-2,6-dicarboxylic acid (4-APDA), 4-phenylpyridine-2,6-dicarboxylic acid (PPDA), 4-(3-nitrophenyl) pyridine-2, 6-dicarboxylic acid (NPPDA), 4-(4-methoxyphenyl)- pyridine-2,6- dicarboxylic acid (MPPDA), 4-(hydroxymethyl)- pyridine-2,6-dicarboxylic acid (4-HMPDA) were prepared according to Refs.[11-14]. The synthetic routes of novel ligands (4-SPDA and DSPDA) are shown in Fig.1.
2.2.1 Preparation of 4-formylpyridine-2,6-dicarboxylic acid (Compound 3)
Compound 1 (11.2 g, 50.0 mmol) was added into dry dichloromethane (50 mL) with pyridinium chloro- chromate(PCC) (11.2 g, 75.0 mmol) at room temperature. After stirring for 5 h, 50% solvent was removed and then ethyl acetate (100 mL) was added. The reaction mixture was washed with water (80 mL), sodium bicarbonate (5%, 80 mL twice) and saturated brine (100 mL) sequentially. The organic layer was collected and dried with Na2SO4, and then concentrated under reduced pressure. The rude solid product was purified by recrystallization (volume ratio of ethyl acetate to chloroform was 3?1) to give Compound 3 as acicular whitish solid (9.6 g). The yield is 86.0% and the melting point range is 152-154 ℃.
IR(KBr), ν/cm-1: 3 037, 2 960, 2 861, 1 734, 1 706, 1 355, 1 213, 978, 781; MS, m/z: 193 (M-OCH3, 58%), 165 (M-OCH3-CO, 100%); 1H-NMR (400 MHz, CDCl3), δ: 10.02 (s, 1H, CHO), 6.64 (s, 2H, Py—H),
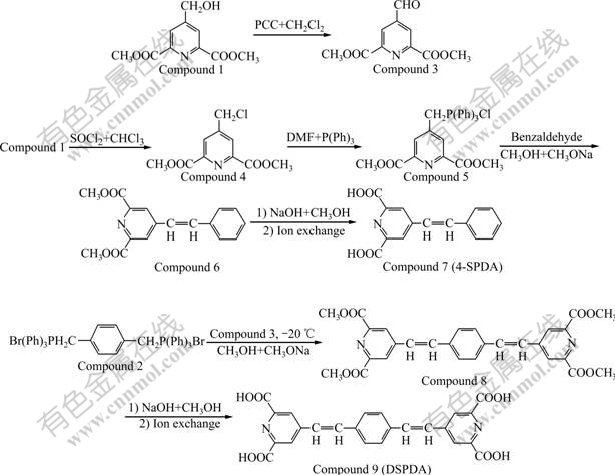
Fig.1 Synthetic route of 4-SPDA and DSPDA
4.06 (s, 6H, OCH3); EA (calculated for C9H10O5N), mass fraction, %: C 53.65 (53.82), H 4.10 (4.06), N 6.30 (6.28).
2.2.2 Preparation of dimethyl 4-(chloromethyl) pyridine- 2,6-dicarboxylate (Compound 4)
Compound 1 (4.5 g, 20.0 mmol) was stirred in anhydrous CHCl3 (25 mL) under nitrogen atmosphere at 0 ℃, sulfuryl dichloride (3.5 g, 30.0 mmol) was added slowly through syringe within 15 min, and the reaction mixture was stirred for an additional 40 min. Then the solvent was removed, the crude product was purified by recrystallization with ethanol to give flaxen solid (Compound 4) with a yield of 82.0% and melting point range of 167-170 ℃.
IR (KBr), ν/cm-1: 3 079, 2 959, 2 836, 1 725, 1 710, 1 380, 1 257, 1 125, 798; MS, m/z: 244 (M+ +1), 213 (M-OCH3, 62%), 184 (M-OCH3-CO, 100%); 1H-NMR (400 MHz, CDCl3), δ: 8.27 (s, 2H, Py—H), 4.45 (s, 2H, CH2Cl), 4.06 (s, 6H, OCH3).
2.2.3 Preparation of dimethyl 4-styrylpyridine-2,6- dicarboxylate (Compound 6)
Triphenylphosphine (6.6 g, 25.0 mmol) was added into a solution of Compound 4 (4.9 g, 20.0 mmol) in dimethyl formamide(DMF, 8 mL). The reaction mixture was stirred under reflux for 2 h, and filtered. The filtrate was refluxed in dry benzene (15 mL) to obtain the crude product Compound 5 (8.6 g) as a colorless solid.
The solution of sodium methanolate (0.4 g, 6.0 mmol) in absolute methanol (5 mL) was added into a absolute methanol (20 mL) containing Compound 5 (2.5 g) and benzaldehyde (0.6 g, 6.0 mmol), through a syringe under nitrogen atmosphere at -20 ℃ within 20 min. The suspension was stirred at -20℃ for 1 h additionally. Then the temperature of reaction was raised to room temperature and kept over night with stirring. 50 mL water was added and the residue was extracted with 50 mL chloroform three times. The organic layer was combined, dried with Na2SO4 and concentrated under reduced pressure. Flash chromatography eluting (volume ratio of ethyl acetate to petroleum ether was 1?3) gave a crude product, which was recrystallized from ethanol to obtain Compound 6 as whitish solid with a yield of 86.5% and melting point range of 195-197 ℃.
IR (KBr), ν/cm-1: 3 120, 2 853, 1 726, 1 638, 1 576, 1 439, 1 255, 1 209, 793, 691; MS, m/z: 297 (M+), 282 (M—CH3, 68%), 238 (M-CH3-CO2, 100%); 1H-NMR (400 MHz, CDCl3), δ: 8.76 (s, 2H, Py—H), 7.68 (d, H, J=16.5 Hz, Py—CH=), 7.62 (d, 1H, J=6.8 Hz, Ph—CH=), 7.68 (m, 5 H, J=3.2 Hz, Ph—H), 3.79 (s, 6H, OCH3); EA (calculated for C17H15O4N), mass fraction, %: C 68.55 (68.68), H 5.10 (5.09), N 4.65 (4.71).
2.2.4 Preparation of 4-styrylpyridine-2, 6-dicarboxylic acid (Compound 7)
A solution of NaOH (30%, 1.1 mL) was added into the mixture of Compound 6 (1.6 g, 5.5 mmol) in methanol (50 mL) and the reaction mixture was stirred at room temperature for 24 h. Evaporation of the solvent gave a white solid, which was purified by 732 cation resin column to give Compound 7 with a yield of 98.4% and melting point range of 283-285 ℃.
1H-NMR (400 MHz, DMSO-d6), δ: 8.241 (s, 2H, Py—H), 7.78 (d, 1H, J=17.0 Hz, Py—CH=), 7.68 (d, 1H, J=16.9 Hz, Ph—CH=), 7.68 (m, 5H, J=3.2 Hz, Ph—H).
2.2.5 Preparation of 4-(4-(2-(2,6-dicarboxypyridin- 4-yl)-vinyl)styryl)pyridine-2,6–dicarboxylic acid (Compound 9)
Compound 8 was obtained following the same procedure described as Compound 6 from Compounds 2 and 3.
Compound 8: Wheat solid, yield 78.3%, and melting point range 223-225 ℃.
IR (KBr), ν/cm-1: 3 448, 2 981, 1 718, 1 654, 1 594, 1 396, 1 288, 964; 1H-NMR (400 MHz, CDCl3), δ: 7.18 (d, 2H, J=16.4 Hz, Ar—CH=), 7.51 (d, 2H, J=16.0 Hz, Py—CH=), 8.36 (s, 4H, Py—H), 7.51 (s, 4H, Ar—H), 3.92 (s, 6H, OCH3); EA (calculated for C24H28O8N2), mass fraction, %: C 65.21 (65.11), H 4.79 (4.86), N 5.30 (5.42).
Compound 9 was obtained following the same procedure described as Compound 7 from Compound 8.
Compound 9: yield 96.1%; 1H-NMR (400 MHz, DMSO-d6), δ: 7.32 (d, 2H, J=17.02 Hz, Ar—CH=), 7.83 (d, 2H, J=16.35 Hz, Py—CH=), 8.46 (s, 4H, Py—H), 7.68 (s, 4H, Ar—H).
2.3 Preparation of complexes of Tb(Ⅲ)
Tb2O3 (3.47 g, 9.48 mmol) was dissolved in a solution of hydrochloric acid (15 mL, 3.00 mol/L), and the mixture was heated to remove superfluous HCl until pH=3 and diluted with redistilled water to 37.5 mL accurately. The concentration of the TbCl3 solution was ascertained by EDTA (11.09 mmol/L) titration using xylenol orange as the indicator.
The solutions of PDA, HPDA, MPDA, 4-APDA, PPDA, NPPDA, MPPDA, 4-HMPDA, 4-SPDA (2.100 mmol) and DPPDA (4.200 mmol) in H2O (37.50 mL) were slowly added into a solution of TbCl3 (0.461 0 mol/L, 1.500 mL) respectively under stirring. Then NaOH (1.00 mol/L) was used to adjust the acidity of the above ten solutions to pH=7 and the mixtures were dried under infrared lamp for 12 h. The complexes of Tb(Ⅲ) were obtained.
2.4 Preparation of solution of Tb3+ complexes
The solution of TbCl3 and the solutions of PDA, HPDA, MPDA, 4-APDA, PPDA, NPPDA, MPPDA, 4-HMPDA, 4-SPDA, DSPDA were all diluted to 0.001 mol/L with redistilled water.
The solution of ligands and the solution of TbCl3 were combined at the volume ratio of 3?1, and the mixture was diluted to 0.01 mmol/L. Ten solutions of complexes were prepared.
3 Results and discussion
3.1 Structural analysis of complexes
The structures of complexes were confirmed by EA and IR data (Table 1). The EA data indicate that the molar ratio of ligand to lanthanide is 3?2 for the DSPDA complex, while the molar ratio of ligand to lanthanide is 3?1 for other complexes. IR data indicate that the asymmetric stretching vibration frequencies (υas) of —COO- in the complexes are shifted downfield by 78-123 cm-1. Meanwhile, the symmetric stretching vibration frequencies (υs) of —COO- are shifted upfield by 11-51 cm-1. From the shifts of IR data, it can be concluded that the ligands all match the lanthanide ions through their carboxyl. The skeleton stretching vibration frequencies of pyridine ring in the complexes (υs, C=N) are lower than those of ligands (150-186 cm-1). This shows that nitrogen atom participates in the chelation. The new peaks appear near 430 cm-1, which suggests that nitrogen atom of the pyridine ring is involved in coordination to the metal centre. On the basis of the above evidence and analysis, it is easily seen that two carboxyls and a nitrogen atom in a pyridine ring of ligand take part in the coordination, and each complex has three double-chelated pyridine rings that have three nitrogen atoms and six oxygen atoms, forming a contorted three coronary triangle prism polyhedral structure[13-14]. So the coordination number of Tb(Ⅲ) is 9. Lanthanide ions in Na6Tb2 (DSPDA)3 also coordinate with 9 atoms because each of two pyridine-2,6- dicarboxylic acid groups has one nitrogen atom in pyridine ring and two carboxyls chelates with a lanthanide ion, which suggests the whole complex may be a polynuclear polymeric netted structure.
3.2 Fluorescence properties of solid complexes
The fluorescence properties of solid complexes are listed in Table 2.
The Tb(Ⅲ) complexes with pyridine-2,6- dicarboxylic acid (PDA) derivatives have kelly fluorescence under ultraviolet. The wavelengths of emission are 491, 543, 582 and 620 nm, corresponding to the 5D4→7F6, 5D4→7F5, 5D4→7F4, 5D4→7F3 transition respectively, and the strongest is 5D4→7F5 among them. Because lanthanide ions have the electronic shell of 5s25p6, which surrounds the electronic shell of 4f, the effect of ligand field in the 4f electronic energy level is very week. So when the ligand is changed, the wavelength of emission is almost unchanged. As we can see from the data, NPPDA and PPDA cannot efficiently sensitize the lanthanide ion, which may be explained by that the electron-withdrawing effect (p-nitrylphenyl in NPPDA and hydroxyl in PPDA) reduces the density of electron cloud in heterocycles and the triplet state cannot match the excitated vibrational-level of lanthanide ion. In addition, the fluorescence intensities of complexes are related to the groups on 4-position of pyridine-2,6- dicarboxylic acid: the fluorescence of the complexes with electron-donating groups is more intense than that of the complexes with electron-drawing groups on 4-position of pyridine ring, i.e. MPDA, 4-APDA>PDA>4-HMPDA, HPDA, MPPDA. Na6Tb2(DSPDA)3·23H2O has more excellent fluorescence properties than other complexes, because the emission energy of ligands is much higher than that of the accepted (Tb3+) and the intersystem crossing (S1→T1) from the first singlet excited state of ligand to the excited triplet state of ligand is increased. Among the ligands, 4-APDA and DSPDA are good sensitizers for Tb(Ⅲ).
Table 1 Yields, EA and IR data of complexes

3.3 Fluorescence properties of complexes in solutions
3.3.1 Effect of pH value on fluorescence properties
H2O was used to dissolve the 10 complexes obtained according to Section 2.3, then solutions of the complexes with concentrations of 0.01 mmol/L were obtained. The effect of pH value on the fluorescence intensity of complexes is listed in Table 3. From the data, it is known that when the pH value of complex solution ranges from 3 to 12, the change tendency for fluorescence intensity of all the complexes is similar. The fluorescence intensity is stronger at pH=7 than that in acidic and alkaline aqueous solutions. Obviously, in the acidic aqueous solution, carboxylate anions can be protonized; while in the alkaline aqueous solution, the lanthanide ions can coordinate with hydroxyl, which produces precipitation as pH value increases gradually. The above two items weaken the chelate ability of the ligands, therefore, the low concentration of the solution results in the decrease of fluorescence intensity of luminescent complexes finally. The similar results about the relationships between the fluorescence intensity of lanthanide complexes of other PDA derivatives and the pH value of the solutions were reported by YIN et al[15].
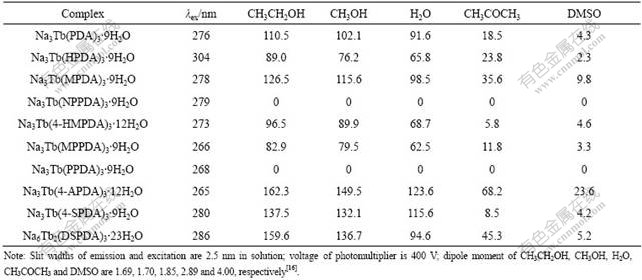
3.3.2 Effect of solvent on fluorescence properties
CH3OH, CH3CH2OH, H2O, CH3COCH3 and dimethyl sulfoxide(DMSO) were used to dissolve the 10 complexes obtained according to Section 2.3, then solutions of the complexes with concentrations of 0.01 mmol/L were obtained. The data of fluorescence intensity in different solvents are listed in Table 4. In conclusion, these results demonstrate clearly that the fluorescence intensities are related to the dipole moments of solvent molecules. Fluorescence intensities decrease as dipole moments of the solvents increase from CH3CH2OH to DMSO. The complexes, which were dissolved in the solvent with lower dipole moment, have stronger fluorescence intensities. SAMY[17] brought forward a theory to explain the phenomenon: the Franck-Condon excited state exists in the system of luminescent molecules. The solvent with lower dipole moment can stabilize the ground state that corresponds to the Franck-Condon excited state. This effect results in that the triplet states of T2(π-π*) exchange with those of T1(π-π*) in ligand. Thus, the transition S(n-π*)→T2(π-π*) becomes possible. And the probability of intersystem crossing (Kiso) from the singlet state to the triplet state of ligand increases[18]. The luminescent mechanism of the ligand→Tb(Ⅲ) (λem=543 nm) is S0→Sh→S1→T→5D4→7F5, and the increase of Kiso causes the enhancement of emissive probability 5D4→7F5 in Tb(Ⅲ), thus, the less the dipole moment of solvent molecule, the stronger the fluorescence intensity.
Table 2 Fluorescence intensity of solid complexes
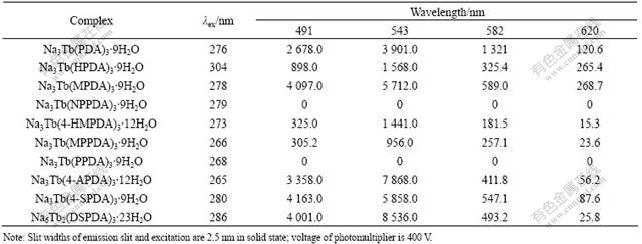
Table 3 Fluorescence intensity of complexes in different pH environments

Table 4 Effect of solvent on fluorescence intensity of complexes
4 Conclusions
1) A series of pyridine-2,6-dicarboxylic acid(PDA) derivatives that contain two novel ligands and their corresponding complexes of Tb(Ⅲ) are obtained and the structural assignment is established on the basis of mass spectrometry and IR, 1H NMR spectrometry.
2) Properties of the binary complexes show: the fluorescence intensities of all the binary complexes of the ligands with Tb(Ⅲ) are strongest when the pH value of the solution is 7. The fluorescence intensity of the complexes with electron-donating groups is more intense than that of the complexes with electron-withdrawing groups. The fluorescence intensity of the complexes in the low-polar solvent is better than that in the high-polar solvent. The complexes with ligands that have polynuclear and larger π-extended conjugated structure have stronger fluorescence intensity
3) 4-Acetaminopyridine-2,6-dicarboxylic acid (4- APDA) and 4-(4-(2-(2,6-dicarboxypyridin-4-yl)- vinyl)styryl)pyridine-2,6-dicarboxylic acid (DSPDA) are good sensitizers for Tb(Ⅲ) ion.
References
[1] WANG Zheng-xiang, SHU Wan-yin, ZHOU Zhong-cheng, LIU You-nian, CHEN Hong. Fluorescence properties and application of doping complexes Eul-XLX (TTA)3 phen as light conversion agents [J]. J Cent South Univ Technol, 2003, 10(4): 342-346.
[2] MUKKALA V M, TAKALO H, LIITTI P, HEMMILA I. Synthesis and luminescence properties of some Eu(Ⅲ) and Tb(Ⅲ) chelate labels having 2,2′:6′,2″-terpyridine as an energy absorbing part [J]. J Alloys Compd, 1995, 225(1): 507-510.
[3] RAU D, MORITA M. Chiral discrimination in circularly polarized luminescence of Tb(Ⅲ)-Nd(Ⅲ) complexes of (S)- and (R)-1,4,7,10- tetraazacyclododecane derivatives [J]. J Lumin, 2001, 94(2): 283- 288.
[4] HA H O, LIM H, CHO W J, HA C S. Improving the efficiency of organic electroluminescent devices by introducing an electron- accepting and thermally stable polymer [J]. Opt Mater, 2002, 21(1): 165-168.
[5] WANG Li-jun, ZHANG Chao-sheng, LI Guo-sheng, LIANG Tao. Geochemical characteristics of rare earth elements in intertidal sediments of Tianjin coastal zone [J]. J Rare Earth, 2002, 20(6): 651-657.
[6] SOKOLNICKI J, LEGENDZIEWICZ J, MULLER G, RIEHL J P. The luminescence, molecular and electronic structure, and excited state energetics of tris-complexes of 4-phenylethynyl-2,6- pyridinedicarboxylic acid with Eu(Ⅲ) and Tb(Ⅲ) prepared in sol-gel [J]. Opt Mater, 2005, 27(9): 1529-1536.
[7] MORITA M, RAU D, HERREN M. Circularly polarized luminescence and enantiomeric energy transfer discrimination of chiral Tb(Ⅲ)-Nd(Ⅲ) EDDS and related complexes [J]. J Alloys Compd, 2004, 380(1): 260-267.
[8] PAUL N W. Synthesis and properties of a twistophane ion sensor: A new conjugated macrocyclic ligand for the spectroscopic detection of metal ions [J]. J Org Chem, 2001, 66(12): 4170-4179.
[9] TANG Rui-ren, ZHENG You-hu, ZHAO Qiang, YAN Zi-er. Synthesis of dimethyl 4-(hydroxylmethyl) pyridine-2,6-dicarboxylate [J]. Chem Reac Eng Technol, 2006, 22(1): 83-87. (in Chinese)
[10] PUSKAS D R, IMRE W, SCHMITT E F. Process for the manufacture of 1,4-bis [2-(4′-carbomethoxystyr- enyl)] benzene: US, 514681 [P]. 1983-07-08.
[11] YIN Xian-hong, YANG Man-ya, SHI Hua-hong, GU Lian-quan. Synthesis of isoquinoline-1, 3-dicarboxylic acid [J]. Chin Chem Lett, 1999, 10(11): 907-910.
[12] TANG Rui-ren, YAN Zi-er, GUO Can-cheng, LUO Li-ming. Synthesis of Eu(Ⅲ) and Tb(Ⅲ) complexes with novel pyridine-2,6- dicarboxylic acid derivatives and their fluorescence properties [J]. Chem J Chin Univ, 2006, 27(3): 472-477. (in Chinese)
[13] MAGYARA A P, SILVERSMITHA A J, BREWERB K S, BOYE D M. Fluorescence enhancement by chelation of Eu3+ and Tb3+ ions in sol-gels [J]. J Lumin, 2004, 108(3): 49-53.
[14] PANIGRAHI B S. Fluorimetric study of terbium, europium and dysprosium in aqueous solution using pyridine carboxylic acids as ligands [J]. J Alloys Compd, 2002, 334(2): 228-231.
[15] YIN Xian-hong, YANG Man-ya, SHI Hua-hong, YANG Guang, CHEN Xiao-ming, GU Lian-quan. Luminescence of terbium chelates with substituted pyridine-2, 6-dicarboxylic acid derivatives by synergistic effect of EDTA [J]. J Chin Rare Earth Soc, 2000, 18(3): 212-215. (in Chinese)
[16] YAO Yong-bing, XIE Tao, GAO Ying-min. Physical chemistry manual [M]. Shanghai: Shanghai Scientific and Technology Press, 1985: 102-103. (in Chinese)
[17] SAMY A E. Spectral, lifetime, fluorescence quenching, energy transfer and photodecomposition of N,N′-bis(2,6-dimethylphenyl)- 3,4:9,10-perylentetracarboxylic diimide (DXP) [J]. Spectrochim Acta Part A, 1999, 55(1): 143-152.
[18] TURRO N J, YAO Shao-ming. The modern molecule photo- chemistry [M]. Beijing: Science Press, 1987: 68-69. (in Chinese)
(Edited by CHEN Wei-ping)
Foundation item: Project(20761002) supported by the National Natural Science Foundation of China
Received date: 2008-02-02; Accepted date: 2008-03-26
Corresponding author: TANG Rui-ren, Professor; Tel: +86-731-8836961; E-mail: trr@mail.csu.edu.cn