铵光卤石热脱水及热分解动力学
周宁波1,肖 华1,陈白珍2,侯朝辉1
(1. 湖南理工学院 化学化工系,湖南 岳阳,414000;
2. 中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:将盐湖水氯镁石溶液和氨法沉镁过滤后的氯化铵溶液混合,在一定条件下制得铵光卤石,研究铵光卤石在热空气中脱水为低水铵光卤石的最佳工艺条件,同时对铵光卤石进行热重-差热分析(TG-DTA)。研究结果表明:铵光卤石在热空气中脱水是分步进行的,包括脱水、水解、热分解等化学反应;在110~180 ℃控制脱水时间可得低水合铵光卤石不水解,超过此温度范围时铵光卤石在脱水的同时会部分发生水解。且铵光卤石于160 ℃脱水4 h可得到含结晶水为0.5~1的低水合铵光卤石,其可用作生产无水氯化镁的原料。用Freeman-Carroll法计算的动力学参数表明,铵光卤石在各温度范围内的脱水反应均不是基元反应,而是由多个不同级数的基元反应所 控制。
关键词:铵光卤石;热脱水;热分解;动力学
中图分类号:O614.23 文献标识码:A 文章编号:1672-7207(2008)02-0311-06
Dehydration and thermal decomposition kinetics on ammonium carnallite in flowing air condition
ZHOU Ning-bo1, XIAO Hua1, CHEN Bai-zhen2, HOU Zhao-hui1
(1. Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,
Yueyang 414000, China;
2. School of Metallurgical Science and Technology, Central South University, Changsha 410083, China)
Abstract: The optimum dehydration technology in hot air atmosphere and thermogravimetry-differential thermal analysis (TG-DTA) curves of ammonium carnallite, which was synthesized using the saline lake bischofite and filtrate stock of deposit magnesium by ammonia as raw materials were studied. The results show that ammonium carnallite will change to dehydrated ammonium carnallite by heating from 110 ℃ to 180 ℃ for controlling dehydration time. But the hydrolysis takes place when temperature is higher than the above temperature. Dehydration process includes dehydration, hydrolysis and thermal decomposition. Dehydrated ammonium carnallite (MgCl2·NH4Cl·nH2O, n=0.5-1) is obtained at 160 ℃ for 4 h, suitable for preparation of anhydrous magnesium chloride. Kinetic parameters calculated by Freeman-Carroll method show that the dehydration of ammonium carnallite is not an elementary reaction, but controlled by a series of elementary reactions in different temperature ranges.
Key words: ammonium carnallite; dehydration; thermal decomposition; kinetics
从水氯镁石的结构中可以看出,在一定外界条件作用下易脱去与Mg2+结合力较弱的4个水,后2个水较难脱去,在较高温度无保护性气氛下脱水会生成氧化镁和碱式氯化镁,即造成水解[1]。为了进一步脱去水氯镁石中的水,可将水氯镁石与氯化物制成复盐。在复盐的晶体结构中,MgCl2与氯化物的结合力要比与水的结合力大,因此,用复盐脱水比水氯镁石脱水容易,且脱水中氯化镁水解的趋势较小[2-4]。在此,本文作者以盐湖水氯镁石溶液与氨法沉镁过滤后的氯化铵溶液为原料合成铵光卤石,铵光卤石在加热条件下脱水为低水合铵光卤石,探索铵光卤石加热脱水的最佳工艺条件,并通过DTA和TG分析研究铵光卤石脱水的动力学行为[5-10]。
1 实 验
1.1 试剂和仪器
试剂有:盐湖卤水;生产镁砂副产的氯化铵溶液。
仪器有:Rigaku 3014型X射线衍射仪;JEOL- 5600LV型扫描电镜;TGA/SDTA851e综合热分析仪;DHG-9076A型电热恒温鼓风干燥箱和DF-101B集热式恒温磁力搅拌器。
1. 2 实验方法
1.2.1 铵光卤石的制备
将青海盐湖产的水氯镁石溶液中的MgCl2与氨法沉镁过滤后的氯化铵溶液中的NH4Cl按摩尔比1?1混合,在pH=6.0,搅拌速度为450 r/min,温度为80~ 85 ℃加热1 h,于低温冷却结晶,过滤,烘干,得铵光卤石(MgCl2?NH4Cl?6H2O)。
1.2.2 铵光卤石脱水
将合成的铵光卤石先通过热分析初步确定铵光卤石的脱水温度范围。在分析天平上称取一定量的铵光卤石平摊于瓷盘(固体的平均厚度不超过1 mm),放置在恒温鼓风干燥箱(温度精度为-0.1~0.1 ℃)中在一定温度范围内加热脱水。
1.2.3 检测与表征方法
采用卡尔费休法分析脱水产物中的水,采用配合滴定法测定脱水产物中氯化镁的含量,采用酸碱滴定法测定脱水产物氧化镁的含量;利用氯化镁易溶于无水乙醇而碱式氯化镁不溶于无水乙醇的性质测定脱水产物中碱式氯化镁;采用综合热分析法确定铵光卤石脱水、水解及热分解温度;用X射线衍射和扫描电镜表征脱水产物的成分和形貌。
2 结果与讨论
2.1 铵光卤石的脱水反应过程
图1所示为铵光卤石的综合热分析曲线。可见,在60~110 ℃有微量的质量损失,其损失量应为颗粒表面吸附水的脱除量;在110~180 ℃,MgCl2·NH4Cl·6H2O脱水为MgCl2·NH4Cl·nH2O,这一区间的质量损失率为37.3%。而MgCl2·NH4Cl·6H2O脱水为MgCl2·NH4Cl·H2O的理论质量损失率为35.1%。这进一步证明MgCl2·NH4Cl·nH2O中的n<1。但在160~180 ℃时铵光卤石在脱水超过一定时间后可能部分发生水解。在180~340 ℃的质量损失是因为MgCl2·NH4Cl·nH2O继续脱水及MgCl2的水解。从铵光卤石的热分析结果看:铵光卤石在热空气中脱水过程包括脱水、水解、热分解等化学反应,其可能的脱水反应可表述如下。
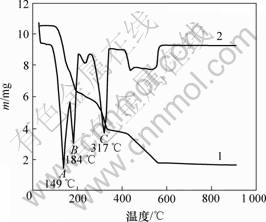
1—TG曲线;2—DTA曲线
图1 铵光卤石的综合热分析曲线
Fig.1 TG-DTA curves of ammonium carnallite
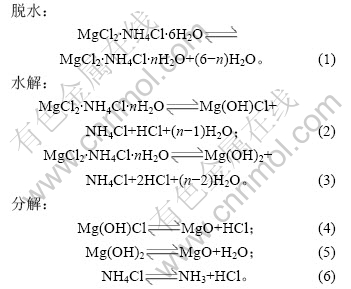
2.2 脱水时间和温度对铵光卤石脱水效果的影响
加热温度和加热时间对铵光卤石的脱水有一定影响,温度越高,铵光卤石脱去的水越多,当温度升高到一定值时,铵光卤石在脱水的同时部分发生水解,且温度越高,水解作用越大;加热时间越长,脱水越完全,但当温度达到一定值时,铵光卤石在脱水的同时水解生成碱式氯化镁或氢氧化镁,此时生成的产物可进一步分解生成氧化镁。表1所示为铵光卤石在不同温度和加热时间的脱水结果(用1 mol铵光卤石加热脱去水的物质的量表示)。
表1 不同温度下脱水时间对铵光卤石脱水效果的影响
Table 1 Effect of temperature and time on dehydration process of ammonium carnallite n(H2O)/mol
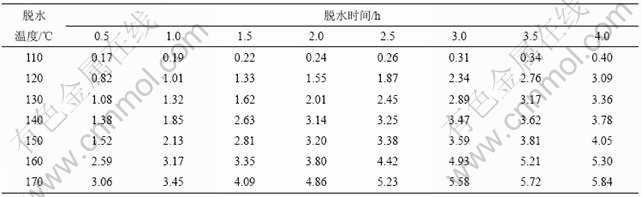
从表1可以看出,在110和120 ℃时,即使加热时间达4 h,铵光卤石只能脱去部分结晶水;在130~150 ℃,随着温度的升高和加热时间的延长,铵光卤石脱去的结晶水越来越多;当温度达到160 ℃,脱水时间为4 h时,铵光卤石脱去的结晶水达5.3个,用这样的低水合铵光卤石采用适当的方法制备的无水氯化镁中氧化镁含量小于0.11%(质量分数),化学分析表明这种低水合铵光卤石中不含碱式氯化 镁[11];但当温度为170 ℃,脱水4 h时,铵光卤石脱去的结晶水为5.84个,经分析,这种低水合铵光卤石中含有0.5%碱式氯化镁。当脱水温度达340 ℃以上时,脱水产物中有氧化镁生成。
图2所示为盐湖原料合成的铵光卤石于160 ℃脱水不同时间的X射线衍射谱。可见,脱水1 h与脱水2 h的谱图差别不大,仅2θ为23.26?,26.68?,29.88?和38.06?处衍射峰的强度不同,证明铵光卤石在160 ℃脱水1 h和2 h时脱去的结晶水的数目差不多。而脱水3 h时,在2θ为55.06?处和脱水4 h时在2θ为69.47?处衍射峰的强度略有增强。
2.3 铵光卤石的脱水反应
反应的动力学方程是由反应机理决定的,一般可写成以下形式[12]:
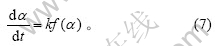
式(7)反映了与α有关的项和与α无关的项可以分离的特殊情况。对于其中的复杂反应,式中的k通常是组成该反应的一些基元反应的速率常数的函数,此时k只是表观速率常数。将Arrhenius公式代入式(7),可以得到

(a) 1 h; (b) 2 h; (c) 3 h; (d) 4 h
图2 铵光卤石于160 ℃时不同脱水时间的XRD谱
Fig.2 XRD patterns of dehydrated ammonium carnallite at 160 ℃ for different times

从上述动力学方程的微分形式出发,可以得到用微商法处理热重曲线的各种表达式。
若反应遵循动力学方程式: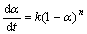 ,此时f(α)=(1-α)n,则有:
,此时f(α)=(1-α)n,则有:

式(13)的左端与?(1/T)/ ?lg(1-α)成线性关系,可由直线的斜率求得表观活化能E,由纵坐标上的截距得到反应级数n,再将E和n代入式(14)可以计算A。此法为Freeman-Carroll法[12-16]。
铵光卤石脱水动力学参数用Freeman-Carroll法计算,铵光卤石的TG-DTA曲线见图1。从TG-DTA曲线可以分别计算各温度下铵光卤石的质量损失率α。设?(1/T)为TG曲线上相邻2点绝对温度的倒数差,dα/dt为TG曲线上各温度下切线的斜率或DTA曲线上各温度点对应的峰强度,以?lg(dα/dt)/?lg(1-α)为纵坐标,?(1/T)/?lg(1-α)为横坐标作图,经线性回归后可得斜率为负的直线。用相关系数r检验直线的线性度,各温度范围内铵光卤石的脱水及热分解表观活化能的计算结果见表2~4和图3~5。
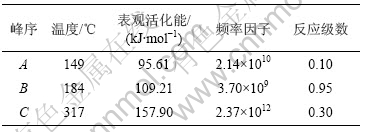
图3~5中各直线的相关系数r均大于0.998 8,直线的斜率等于-E/(2.303R),由此可以计算各温度范围内的表观活化能E,直线的截距为反应级数n,将E和n分别代入式(14),可得频率因子A,再将E和A代入Arrhenius方程k=Ae-E/(RT),可得温度为T时的k。铵光卤石在不同温度范围内的脱水及热分解动力学参数见表5。
表2 铵光卤石A峰表观脱水活化能的计算过程
Table 2 Calculating process for activation energy of peak A on dehydration of ammonium carnallite
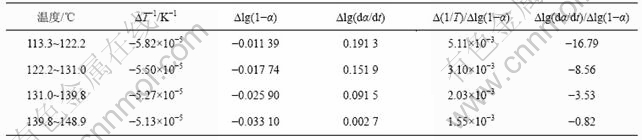
表3 铵光卤石B峰脱水及水解表观活化能的计算过程
Table 3 Calculating process for activation energy of peak B on dehydration and hydrolysis of ammonium carnallite
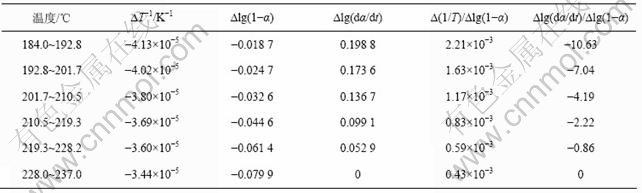
表4 铵光卤石C峰水解及热分解表观活化能的计算过程
Table 4 Calculating process for activation energy of peak C on hydrolysis and decomposition of ammonium carnallite

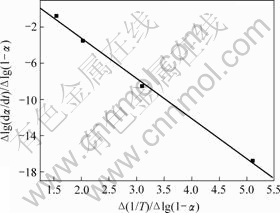
图3 铵光卤石A峰脱水表观活化能的计算图
Fig.3 Calculating graph for activation energy of peak A on dehydration of ammonium carnallite

图4 铵光卤石B峰脱水及水解表观活化能的计算图
Fig.4 Calculating graph for activation energy of peak B on dehydration and hydrolysis of ammonium carnallite
从表5可以看出,A峰的表观活化能相对较小。表观活化能越小,反应越易进行,由此说明在此温度下铵光卤石脱水较易进行。B峰的表观活化能比A峰的表观活化能增大约14%,即随着温度的升高,铵光卤石的脱水变得越来越难。C峰的表观活化能最大。此时,随着温度进一步升高,主要进行的是水解反应和部分物质的热分解反应。水解反应和热分解反应的表观活化能比脱水反应的表观活化能高。此外,各温度下反应级数都不为整数,说明铵光卤石的脱水及热分解并非基元反应,而是由多个基元反应所控制。

图5 铵光卤石C峰水解及热分解表观活化能的计算图
Fig.5 Calculating graph for activation energy of peak C on hydrolysis and decomposition of ammonium carnallite
表5 铵光卤石在不同温度下的脱水及热分解过程动力学参数
Table 5 Dehydration and decomposition kinetic parameters of ammonium carnallite at different temperatures
3 结 论
a. 在110~180 ℃温度范围内控制脱水时间,铵光卤石变成低水合铵光卤石(MgCl2·NH4Cl·nH2O)而不水解;高于180 ℃时,铵光卤石在脱水的同时会部分发生水解作用,高温时甚至会发生热分解。
b. 铵光卤石的脱水过程包括脱水、水解、热分解等化学反应。
c. 脱水温度和时间对铵光卤石的脱水效果有较大的影响:若温度低,则铵光卤石脱水不完全;若温度高,则铵光卤石在脱水的同时会发生水解作用。
d. 用Freeman-Carroll法计算了铵光卤石脱水的动力学参数表观活化能E、反应级数n、频率因子A。从这些动力学参数可以判断在不同温度下铵光卤石脱水反应的难易程度。各温度范围下的反应级数n均不为整数,说明铵光卤石脱水反应并非基元反应,必定是由多个不同级数的基元反应所控制。
参考文献:
[1] 徐日瑶. 有色金属提取手册: 镁分册[M]. 北京: 冶金工业出版社, 1992.
XU Ri-yao. A handbooks for extractive metallurgy of nonferrous metals: Magnesium[M]. Beijing: Metallurgical Industry Press, 1992.
[2] 杨国良. 水氯镁石与废电解质制备炼镁用钾光卤石工艺的探讨[J]. 铝镁通讯, 1999(3): 42-44.
YANG Guo-liang. Study on preparation of sylvite carnallite from bischofite and waste electrolyte[J]. Correspondence of Aluminum and Magnesium, 1999(3): 42-44.
[3] ZHOU Ning-bo, CHEN Bai-zhen, HE Xin-kuai, et al. Preparation and characterization of anhydrous magnesium chloride with dehydrated ammonium carnallite[J]. Journal of Central South University of Technology, 2006, 13(4): 373-378.
[4] 周宁波, 陈白珍, 何新快, 等. 铵光卤石气固反应法制备无水氯化镁[J]. 应用化学, 2005, 22(8): 874-878.
ZHOU Ning-bo, CHEN Bai-zhen, HE Xin-kuai, et al. Preparation and characterization of anhydrous magnesium chloride in reaction of gas-solid phase by ammonium carnallite[J]. Journal of Applied Chemistry, 2005, 22(8): 874-878.
[5] Arvanitidis I, Sichen D, Seetharaman S. A study of the thermal decomposition of BaCO3[J]. Metallurgical and Materials Transations B, 1996, 27B: 409-416.Mohamed A M, Samih A H. Non-isothermal decomposition of cadmium monohydrate in different atmospheres[J]. Journal of Analytical and Applied Pyrolysis, 2002, 65: 287-300.
[6] Mohamed A M ,Samih A H. Non-isothermal decomposition of cadmium monohydrate in different atmospheres[J]. Journal of Analytical and Applide Pyrolysis, 2002,65:287-300
[7] Hu H P, Chen Q Y, Yin Z L, et al. Thermal behaviors of mechanically activated pyrites by thermogravimetry(TG)[J]. Thermochimica Acta, 2003, 398: 233-240.
[8] Robson F F. Solid state synthesis characterization and thermogravimetric study of the adducts CoCl2·6L (L=ethyleneurea, propyleneurea and ethylenethiourea)[J]. Thermochimica Acta, 2001, 376: 63-66.
[9] Arvanitidis I, Sichen D, Sohn H Y. The intrinsic thermal decomposition kinetics of SrCO3 by a nonisothermal technique[J]. Metallurgical and Materials Transations B, 1997, 28B: 1063-1068.
[10] Vadim M, Serge B. Modulated thermogravimetry in analysis of decomposition kinetics[J]. Chemical Engineering Science, 2005, 60: 747-766.
[11] 周宁波, 陈白珍, 何新快. 有机溶剂无水氯化镁的制备与表征[J]. 无机化学学报, 2005, 21(2): 286-290.
ZHOU Ning-bo, CHEN Bai-zhen, HE Xin-kuai. Preparation and characterization of anhydrous magnesium chloride in organic solvent[J]. Journal of Inorganic Chemistry, 2005, 21(2): 286-290.
[12] 蔡正千. 热分析[M]. 北京: 高等教育出版社, 1993.
CAI Zheng-qian. Thermal analysis[M]. Beijing: Higher Education Press, 1993.
[13] 胡祖容, 史启祯. 热分析动力学[M]. 北京: 科学出版社, 2001.
HU Zou-rong, SHI Qi-zhen. Kinetics of thermal analysis[M]. Beijing: Science Press, 2001.
[14] Shuto Y, Nobuyoshi K. Kinetics of the thermal decomposition of sodium hydrogencarbonate evaluated by controlled rate evolved gas analysis coupled with thermogravimetry[J]. Thermochimica Acta, 2005, 431: 38-43.
[15] Gao P Z, Wang H J, Jin Z H. Study of oxidation properties and decomposition kinetics of three-dimensional(3-D) braided carbon fiber[J]. Thermochimica Acta, 2004, 414: 59-63.
[16] Mayoral M C, Izquierdo M T, Andres J M, et al. Different approaches to proximate analysis by thermogravimetry analysis[J]. Thermochimica Acta, 2001, 370: 91-97.
收稿日期:2007-06-05;修回日期:2007-07-26
基金项目:湖南省自然科学基金资助项目(07JJ3096)
通信作者:周宁波(1962-),男,湖南岳阳人,博士,副教授,从事冶金物理化学与材料化学研究;电话:0730-8856393;E-mail: znb8787@163.com