ISOTHERMAL KINETICS OF THE FIRST STAGE REDUCTION OF MAGNETITE COLD BOND PELLETS WITH CARBON MONOXIDE
来源期刊:中南大学学报(英文版)1998年第1期
论文作者:Chen Qiyuan Zhou Hong Zhang Pingmin Qiu Guanzhou Xu Jing
文章页码:2 - 3
Key words:kinetics; thermogravimetry; reduction; cold bond pellet
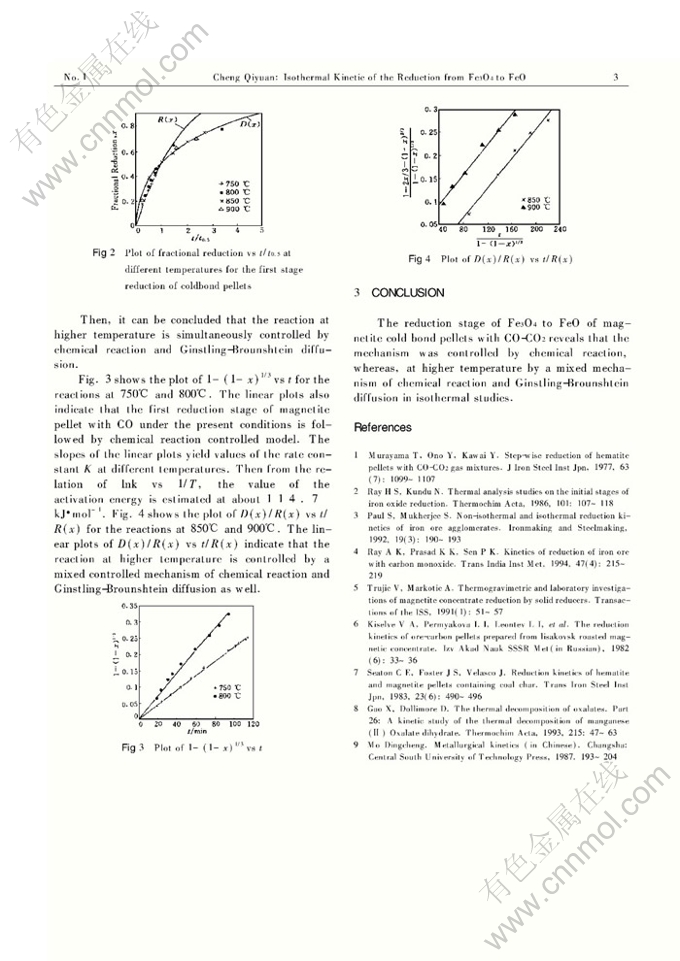
Abstract: The reduction of Fe3O4 to Fe is accomplished in two stages. In order to understand the reduction mechanism of magnetite cold bond pellets during the first period of heating-up process, it is necessary to study the kinetics of the reaction. In this work, the first stage of reduction of magnetite cold bond pellets, that is the reduction from Fe3O4 to FeO, has been investigated using an isothermal thermogravimetric method. Isothermal experiments were carried out at 750℃, 800℃, 850℃and 900℃, respectively. The results showed that the reaction was controlled by chemical reaction below 800℃, whereas, at higher temperature, it was controlled by a mixed controlled mechanism of chemical reaction and Ginstling-Brounshtein diffusion as well.