文章编号:1004-0609(2010)12-2438-07
Te(Ⅳ)-H2SO4 -H2O体系中卤素离子催化还原
Te(Ⅳ)反应动力学
孙召明,郑雅杰
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:以卤素离子(Cl-、Br-、I-)为催化剂,对SO2还原Te(Ⅳ)反应动力学进行研究。结果表明:在高浓度硫酸体系中,卤素离子(Cl-、Br-、I-)的浓度为0.3 mol/L,SO2流量为18 L/h,温度为90 ℃,Cl-、Br-和I-的反应时间分别超过60、10、10 min时,Te(Ⅳ)还原率均为97%。以Cl-和Br-为催化剂时,还原产物为单质碲;I-为催化剂时,还原产物为TeI。Cl-催化还原Te(Ⅳ)动力学表明,反应速率与Te(Ⅳ)浓度的成正比,该化学反应属于准一级反应。反应活化能受Cl-浓度的影响,当Cl-浓度为0.1和0.3 mol/L时,其活化能分别为44.871和36.714 kJ/mol。
关键词:碲;卤素离子;催化;还原;动力学
中图分类号:TQ125.3 文献标志码:A
Reaction kinetics of Te(Ⅳ) using halogen ions as catalyst in
Te(Ⅳ)-H2SO4-H2O system
SUN Zhao-ming, ZHENG Ya-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The reaction kinetics of Te(Ⅳ) reduced via SO2 were investigated with halogen ions(Cl-, Br- and I-) as catalyst. The results show that the reduction ratios of Te(Ⅳ) in the high concentration system of sulfuric acid are all more than 97% after Te(Ⅳ) is reduced when the reaction times of Cl-, Br- and I- are over 60, 10 and 10 min under the conditions of c(Cl-), c(Br-) and c(I-) of 0.3 mol/L, SO2 flow rate of 18 L/h and temperature of 90 ℃. The reduction product of Te(Ⅳ) is elemental tellurium using Cl- or Br- as catalyst, and the reduction product is TeI using I- as catalyst. The reaction kinetics of Te(Ⅳ) using Cl- as catalyst indicates that the reaction rate is proportional to the concentration of Te(Ⅳ), and this chemical reaction is in accordance with pseudo-first-order reaction. The reaction activation energy is influenced by the concentration of chloride ion. When the concentrations of chloride ion are 0.1 and 0.3 mol/L, the activation energy are 44.871 and 36.714 kJ/mol, respectively.
Key words: tellurium; halogen ion; catalysis; reduction; kinetics
作为当代高新材料的支撑材料,碲被广泛用于电子信息[1-5]、冶金[6]、化工[7-8]、医药卫生[9]、宇航[10-11]和能源[12]等工业领域。碲的原料主要来源于铜冶炼过程产生的阳极泥。目前,回收碲的方法主要有铜粉置换法[13]、中和沉淀法[14]和SO2还原法。研究发现,当溶液中硫酸浓度不小于880 g/L时,SO2无法还原溶液中的Te(Ⅳ)。郑雅杰等[15]采用卤素离子催化还原法回收了高浓度硫酸液中的碲和铜,已申请国家专利,该技术在大型有色企业已得到应用。该方法与铜粉置换法相比,不仅节约了铜粉,而且回收了高浓度硫酸溶液中的铜;与中和沉淀法比较,该方法成本低,产品中碲含量高、杂质含量低。研究高浓度硫酸体系下卤素离子催化还原Te(Ⅳ)的动力学有助于深入了解反应过程中卤素离子的催化作用机理,为工业生产优化提供指导。在此,本文作者考查卤素离子对Te(Ⅳ)- H2SO4-H2O的体系中Te(Ⅳ)还原效果的影响,并对Cl-催化还原Te(Ⅳ)动力学进行研究。
1 实验
1.1 实验原料
原料为H2SO4(AR)、HNO3(AR)、超纯水、NaCl(AR)、NaBr(AR)、KI(AR)、SO2(99.95%)、碲粉。碲粉的成分如表1所列。
表1 碲粉成分
Table 1 Contents of tellurium powder (mass fraction, %)

1.2 实验步骤
取表1所列成分的碲粉,用硝酸氧化后加入硫酸液溶解并煮沸驱赶硝酸,冷却后加水配制成H2SO4浓度均为880 g/L,而Te(Ⅳ)浓度不同的溶液备用。实验时,取400 mL含Te(Ⅳ)液加入到1 L三颈烧瓶中,利用恒温水浴将含Te(Ⅳ)液加热至反应温度并保持温度恒定,然后根据卤素离子的种类和浓度向含Te(Ⅳ)液中分别加入相应量的固体卤化物,搅拌20 min后,取样。紧接着通入二氧化硫还原,在反应过程中随时间变化取样、分析。
1.3 分析与检测
溶液中Te(Ⅳ)浓度利用电感耦合等离子体发射光谱仪(ICP,Intripid Ⅱ XSP)进行检测,产物物相通过X-射线衍射仪(XRD, D/max-TTRⅢ)分析。
2 结果与讨论
2.1 Cl-为催化剂时的反应动力学
溶液中H2SO4浓度为880 g/L、Te(Ⅳ)的浓度为7.0 g/L,Cl-的浓度为0.3 mol/L。当SO2流量为18 L/h时,不同温度下的反应时间对Te(Ⅳ)浓度的影响如图1 所示。
将反应时间及相应Te(Ⅳ)浓度代入气-液反应动力学公式[16]:
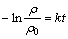 (1)
(1)
式中:ρ为溶液中Te(Ⅳ)的浓度,g/L;ρ0为原始溶液中Te(Ⅳ)的浓度,g/L;k为化学反应速率常数,min-1; t为反应时间,min。
-ln(ρ/ρ0)—t的关系如图2所示。由图2可知,-ln(ρ/ρ0)与t具有较好的线性关系,表明该动力学符合上述气-液化学反应规律。
SO2进入溶液后发生如下反应:
SO2+H2O=H2SO3
2H2SO3+TeCl4+2H2O=2H2SO4+4HCl+Te↓
上述两个反应为主要反应,还原速度受这两步影响,其反应速度方程分别为
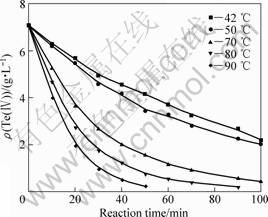
图1 不同温度下反应时间对Te(Ⅳ)浓度的影响
Fig.1 Effect of reaction time on concentration of Te(Ⅳ) at different reaction temperatures

图2 不同反应温度下-ln(ρ/ρ0)与t的关系
Fig.2 Relationship between -ln(ρ/ρ0) and reaction time at different reaction temperatures


式中:ρH2SO3、ρTe(Ⅳ) 、……为参与反应各种物质的浓度,上标n1、n2、……为反应物的反应级数,k+、k-分别为正、逆反应的速率常数。反应前、后ρH2O、ρH2SO4、ρCl-基本不变,反应过程中H2SO3浓度恒定,因此速度方程可表示为:
 (2)
(2)
由反应速率方程(1)和(2)可知,n2等于1,说明该反应属于准一级反应(Pseudo-first order reaction)[17]。
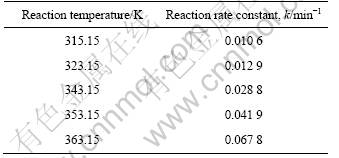
根据图2中各直线斜率,求出不同温度下的反应速率常数k值,如表2所列。
表2 Cl-浓度为0.3 mol/L时不同温度下的反应速率常数k
Table 2 Reaction rate constant k under different temperatures at Cl- concentration of 0.3 mol/L
根据表2数据作出lnk与1/T的关系图,如图3所示。图3中拟合直线斜率为-4 416,即-Ea/R=-4 416,求得活化能Ea=36.714 kJ/mol(<40 kJ/mol),说明SO2还原Te(Ⅳ)受扩散控制。
硫酸浓度不变,改变Te(Ⅳ)和Cl-浓度进行实验。溶液成分为H2SO4 880 g/L,Te(Ⅳ) 3.96 g/L、Cl- 0.1 mol/L。
当Cl-浓度为0.1 mol/L时,不同反应温度下,Te(Ⅳ)浓度随反应时间的变化如图4所示。由图4和图1可知,即使Cl-浓度和Te(Ⅳ)浓度不同,但体现的反应规律一致。
将实验数据代入一级反应速度方程,-ln(ρ/ρ0)-t的关系如图5所示。由图5可知,Cl-浓度为0.1 mol/L时,-ln(ρ/ρ0)与t符合直线关系。不同温度下反应速率常数k如表3所列。
由表3数据,作lnk—1/T图,结果如图6所示。
由图6可知,lnk与1/T成线性关系,拟合直线的斜率为-5 397,即-Ea/R=-5 397,求得活化能为44.871

图3 Cl-浓度为0.3 mol/L时lnk与1/T的关系
Fig.3 Relationship between lnk and 1/T at Cl- concentration of 0.3 mol/L

图4 Cl-浓度为0.1 mol/L时反应时间对Te(Ⅳ)浓度的影响
Fig.4 Effect of reaction time on Te(Ⅳ) concentration at Cl- concentration of 0.1 mol/L
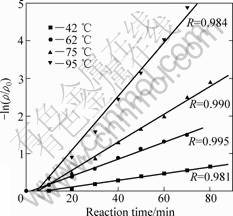
图5 -ln(ρ/ρ0)和反应时间t的关系
Fig.5 Relationship between -ln(ρ/ρ0) and reaction time
表3 Cl-浓度为0.1 mol/L时不同温度下反应速率常数k
Table 3 Reaction rate constant k at different temperatures under Cl- concentration of 0.1 mol/L


图6 lnk和反应温度的关系
Fig.6 Relationship between lnk and reaction temperature
kJ/mol(>40 kJ/mol),反应受化学反应控制。
研究表明,Cl-浓度为0.3 mol/L时的活化能比Cl-浓度为0.1 mol/L时的小,说明提高Cl-浓度可以降低反应活化能。
Cl-对SO2还原Te(Ⅳ)催化作用在于改变了Te(Ⅳ)在溶液中的存在形态。在添加Cl-后,体系中Te(Ⅳ)存在形态发生以下变化:

Te(Ⅳ)形态的改变破坏了Te=O双键的稳定性并降低了 的阻碍作用,从而有利于还原反应的进行。当Te(Ⅳ)完全与Cl-形成TeCl4时,反应速度决定于溶液中H2SO3的浓度。因此,当Cl-浓度为0.3 mol/L时,反应受扩散控制,其活化能较低,易于反应。当Te(Ⅳ)不能完全与Cl-形成TeCl4时,反应受化学反应控制,其活化能高,反应慢。
的阻碍作用,从而有利于还原反应的进行。当Te(Ⅳ)完全与Cl-形成TeCl4时,反应速度决定于溶液中H2SO3的浓度。因此,当Cl-浓度为0.3 mol/L时,反应受扩散控制,其活化能较低,易于反应。当Te(Ⅳ)不能完全与Cl-形成TeCl4时,反应受化学反应控制,其活化能高,反应慢。
当Cl-浓度为0.3 mol/L时,SO2还原产物的XRD谱为图7中上部分,其SEM像如图8所示。由图7可知,在高浓度硫酸体系下中,Cl-为催化剂时,SO2还原Te(Ⅳ)的产物为单质碲。由图8可知,还原固体产物晶型为立方体。

图7 不同催化剂作用下 SO2还原产物的XRD谱
Fig.7 XRD patterns of products reduced by SO2 with different catalysts: (a) Cl-; (b) Br-; (c) I-

图8 Cl-浓度为0.3 mol/L时 SO2还原产物的SEM像
Fig.8 SEM image of product reduced by SO2 at Cl- concentration of 0.3 mol/L
2.2 Br-为催化剂时的反应动力学
用浓硝酸氧化碲粉后加入硫酸溶液,加热驱赶硝酸,配制成含Te(Ⅳ)的硫酸溶液。每次取400 mL溶液于三颈瓶中,并置于恒温水浴上加热、恒温。向溶液中加入KBr搅拌20 min,然后以18 L/h流量向溶液中通入SO2,还原。
当Br-和Te(Ⅳ)浓度分别为0.3 mol/L和6.65 g/L时,反应时间对Te(Ⅳ)浓度的影响如图9所示。Br-浓度为75 mmol/L、Te(Ⅳ) 浓度为5.01 g/L时,反应时间对Te(Ⅳ)浓度的影响如图10所示。
由图9可知,在NaBr浓度为0.3 mol/L时,Te(Ⅳ)还原速度随反应温度的升高而加快。当反应时间不长于10 min时,Te(Ⅳ)浓度随反应时间的延长呈直线下降,Te(Ⅳ)被迅速还原;当反应时间不短于15 min时,受扩散传质影响,反应速度减慢。
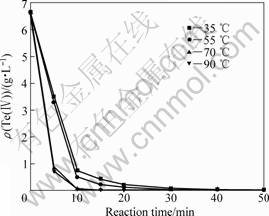
图9 KBr浓度为0.3 mol/L时反应时间对Te(Ⅳ)浓度的影响
Fig.9 Effect of reaction time on Te(Ⅳ) concentration at KBr concentration of 0.3 mol/L

图10 KBr浓度为75 mmol/L时反应时间对Te(Ⅳ)浓度的影响
Fig.10 Effect of reaction time on Te(Ⅳ) concentration at KBr concentration of 75 mmol/L
由图10可知,当NaBr浓度为75 mmol/L时,Te(Ⅳ)还原速度随反应温度的升高而减慢。当反应时间不长于5 min时,Te(Ⅳ)浓度下降缓慢;当反应时间不短于10 min时,Te(Ⅳ)浓度随反应时间的延长而降低。
由于残留HNO3及其化合物的影响,高温下Br-易于氧化为Br。Br-浓度越低,氧化后溶液中起催化作用的Br-浓度越低,对Te(Ⅳ)还原速度的影响越大,导致反应速度过慢。实验现象也表明,反应前期有红棕色Br2产生。
Br-催化还原时,存在如下反应:
2NaBr+H2SO4=Na2SO4+2HBr
4HBr+TeOSO4=TeBr4+H2SO4+H2O
TeBr4+2H2SO3+2H2O=Te+2H2SO4+4HBr
当Br-浓度为0.3 mol/L、SO2气流量为18 L/h时,还原产物洗涤后的XRD谱如图7(b)所示。由图7(b)可知,用NaBr为催化剂时,还原产物为单质碲,与NaCl为催化剂时的产物相同。
2.3 I-为催化剂时的反应动力学
溶液成分:H2SO4 880 g/L、Te(Ⅳ)浓度7.18 g/L。取400 mL溶液于三颈瓶中,置于恒温水浴器上加热并恒温,加入KI固体。搅拌20 min后取样分析溶液中Te(Ⅳ),然后向溶液中通入18 L/h SO2、还原。I-初始浓度分别为0.3和0.1 mol/L时,加入KI 20 min后,温度对溶液Te(Ⅳ)的影响如表4所列。
表4 不同KI浓度下温度对Te(Ⅳ)浓度的影响
Table 4 Effect of temperature on Te(Ⅳ) concentration under different KI concentrations
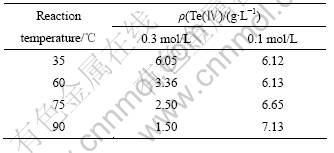
由表4可知,Te(Ⅳ)被KI还原。在高浓度I-作用下,Te(Ⅳ)还原速度随温度的升高而加快,Te(Ⅳ)还原率由15.7%提高到79.1%;在低浓度I-作用下,其反应速度随温度的升高而减慢。由于高温加速了I-的氧化,降低了溶液中起还原和催化作用的I-浓度,在I-初始浓度较低时,Te(Ⅳ)还原率随温度升高而降低;当I-初始浓度较高时,I-浓度相应受氧化作用影响小,升高温度加快了Te(Ⅳ)还原速度,还原率随温度的升高而增大。
与KI反应20 min后,通SO2还原,反应时间对Te(Ⅳ)浓度的影响如图11和12所示。
由图11可知,当I-浓度高时,通入SO2 10 min后,溶液中Te(Ⅳ)迅速下降,Te(Ⅳ)总还原率达到97%,表明碘可以加速Te(Ⅳ)的还原。催化还原速度受反应温度影响明显,随温度的升高而下降。
由图12可知,Te(Ⅳ)还原率随反应时间的延长而

图11 KI浓度为0.3 mol/L时反应时间对Te(Ⅳ)浓度的影响
Fig.11 Effect of reaction time on Te(Ⅳ) concentration at KI concentration of 0.3 mol/L
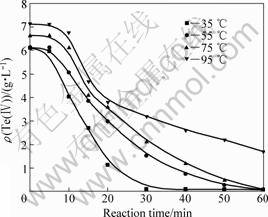
图12 KI浓度为0.1 mol/L时反应时间对Te(Ⅳ)浓度的影响
Fig.12 Effect of reaction time on Te(Ⅳ) concentration at KI concentration of 0.1 mol/L
增大。当KI浓度为0.1 mol/L时,碲还原速度随温度升高而减慢,与I-高浓度情况下规律相同。
将SO2通入KI溶液后,有以下化学反应存在:
SO2+2H2O+I2=H2SO4+2HI
8HI+2TeOSO4=2TeI+3I2+2H2SO4+2H2O
反应产生的HI进一步促进Te(Ⅳ)还原为Te(Ⅰ)。当KI浓度为0.1 mol/L时,在I-的作用下,SO2还原产物中存在TeI。
I-最外层电子排布为5s25p6,Te(Ⅳ) 最外层电子排布为5s25p0。由于I-半径大,极易失去电子,当I-遇到Te(Ⅳ)时,I-将p轨道上多余电子传递到Te(Ⅳ)的空p轨道,I-转化为单质I2, Te(Ⅳ)转化为最外层电子排布为5s25p3的Te+,p层达到半充满的稳定状态。Te+与I-结合,形成不溶于高浓度硫酸溶液的TeI。I-在反应过程中实际起到传递电子和沉淀剂的作用。
3 结论
1) 用Cl-为催化剂显著提高了SO2还原Te(Ⅳ)的速度和回收率,还原产物为单质碲。当 Cl-浓度为0.3 mol/L,二氧化硫流量为18 L/h,反应温度为90 ℃,反应时间为60 min时,Te(Ⅳ)还原率为97%。以Cl-为催化剂时,还原速度与溶液中Te(Ⅳ)浓度成正比,化学反应属准一级反应。当Cl-浓度为0.1 mol/L时,反应活化能为44.871 kJ/mol;当Cl-浓度为0.3 mol/L时,反应活化能为36.714 kJ/mol,表明提高Cl-浓度可降低反应活化能。
2) 以Br-为催化剂显著提高了SO2还原Te(Ⅳ)的速度和回收率,还原产物为单质碲。当 Br-浓度为0.3 mol/L,二氧化硫流量为18 L/h,反应温度为90 ℃,反应时间为10 min时,Te(Ⅳ)还原率为97%。
3) 以I-为催化剂时,I-可直接还原溶液中Te(Ⅳ),还原产物为TeI。当I-浓度为0.3 mol/L时,反应温度为90 ℃,加入KI 20 min后,Te(Ⅳ)还原率达到79%。与KI反应20 min后,通入SO2。当SO2其流量为18 L/h、反应时间为10 min时,Te(Ⅳ)总还原率为97%。
REFERENCES
[1] SICILIANO T, DIGIULIO M, TEPORE M, FILIPPO E, MICOCCI G, TEPORE A. Ammonia sensitivity of RF sputtered tellurium oxide thin films[J]. Sensors and Actuators B, 2009, 138(2): 550-555.
[2] TSIULYANU D, TSIULYANU, LIESS H D, EISELE I. Characterization of tellurium-based films for NO2 detection[J]. Thin Solid Films, 2005, 485(1/2): 252-256.
[3] 叶振华, 周文洪, 胡伟达, 胡晓宁, 丁瑞军, 何 力. 碲镉汞红外双色探测器响应光谱研究[J]. 红外与毫米波学报, 2009, 28(1): 4-6.
[4] YE Zhen-hua, ZHOU Wen-hong, HU Wei-da, HU Xiao-ning, DING Rui-jun, HE Li. Spectral study on response of HgCdTe IR two color detector arrays[J]. Journal of Infrared and Millimeter Waves, 2009, 28(1): 4-6.
[5] LIANG Feng-xia, QIAN Hai-sheng. Synthesis of tellurium nanowires and their transport property[J]. Materials Chemistry and Physics, 2009, 113(2/3): 523-526.
[6] SONG H, CHO K, KIM H, LEE J S, MIN B, KIM H S, KIM S W, NOH T, KIM S. Synthesis and characterization of nanocrystalline mercury telluride by sonochemical method[J]. Journal of Crystal Growth, 2004, 269(2/4): 317-323.
[7] 袁武华, 王 峰. 国内外易切削钢的研究现状和前景[J]. 钢铁研究, 2008, 36(5): 56-57.
[8] YUAN Wu-hua, WANG Feng. Present research status and prospects on free cutting steel at home and abroad[J]. Research on Iron & Steel, 2008, 36(5): 56-57.
[9] NICOLA PETRAGNANIA, HE’LIO A, STEFANIB. Advances in organic tellurium chemistry[J]. Tetrahedron, 2005, 61(7): 1613-1679.
[10] 黄 宪, 杜灿屏. 有机硒碲化合物在高选择性合成反应中的应用[J]. 化学通报, 1999(9): 21-25.
[11] HUANG Xian, DU Can-ping. The application of organoselenium compounds and organo-telluronium compounds in the high-selected synthesis reaction[J]. Chemistry, 1999(9): 21-25.
[12] 李新军, 叶长芸, 张晶波, 卢 珊, 白雪梅, 徐建国. 产志贺毒素大肠杆菌O157:H7的亚碲酸钾抗性基因簇分布和抗性水平[J]. 中华微生物学和免疫学杂志, 2003, 23(11): 833-836.
[13] LI Xin-jun, YE Chang-yun, ZHANG Jing-bo, LU Shan, BAI Xue-mei, XU Jian-guo. Distribution of tellurite resistance gene cluster and its resistance level in Stx-positive E.coli O157:H7[J]. Chinese Journal of Microbiology and Immunology, 2003, 23(11): 833-836.
[14] 涂锋华, 王维扬, 董德平, 付立英. 压电陶瓷微位移驱动器在FY-3卫星G型辐射制冷器上的应用[J]. 科学技术与工程, 2008, 8(14): 4029-4030.
[15] TU Feng-hua, WANG Wei-yang, DONG De-ping, FU Li-ying. Piezoelectric ceramic actuators applied to G-radiant cooler for FY-3 meteorological satellite[J]. Science Technology and Engineering, 2008, 8(14): 4029-4030.
[16] 马玉天, 龚竹青, 徐卫红. 化学气相沉积法制备光谱选择性碲膜[J]. 中南大学学报: 自然科学版, 2006, 37(5): 908-912.
[17] MA Yu-tian, GONG Zhu-qing, XU Wei-hong. Preparation of tellurium films with spectral selectivity by chemical vapour deposition method[J]. Journal of Central South University: Science and Technology, 2006, 37(5): 908-912.
[18] TAKASHIRI M, SHIRAKAWA T, MIYAZAKI K, TSUKAMOTO H. Fabrication and characterization of bismuth-telluride-based alloy thin film thermoelectric generators by flash evaporation method[J]. Sensors and Actuators A, 2007, 138(2): 329-334.
[19] 刘兴芝, 宋玉林, 武荣成, 熊 英, 朗 红, 臧树良. 碲化铜法回收碲的物理化学原理[J]. 广东有色属学报, 2002, 12(s1): 55-57.
[20] LIU Xing-zhi, SONG Yu-lin, WU Rong-cheng, XIONG Ying, LANG Hong, ZANG Shu-liang. Physicochemical principle for recovering tellurium by copper telluride method[J]. Journal of Guangdong Non-ferrous Metal, 2002, 12(s1): 55-57.
[21] 阮胜寿, 汪劲松. 碱中和法从分铜液中提碲的试验研究[J]. 有色金属(冶炼部分), 2004(1): 31-32.
[22] RUAN Sheng-shou, WANG Jing-song. Study on tellurium extracting from copper leached solution by alkali neutralization process[J]. Nonferrous Metals (Extractive Metallurgy), 2004(1): 31-32.
[23] 郑雅杰, 孙召明, 汪 蓓, 滕 浩, 洪 波. 阳极泥预处理及回收稀散金属的方法: 中国, 200810032022.0[P]. 2008-08-08.
[24] ZHENG Ya-jie, SUN Zhao-ming , WANG Bei, TENG Hao, HONG Bo. The pretreatment of copper refinery slime and the method of recovery rare elements: CN, 200810032022.0[P]. 2008-08-08.
[25] 李洪桂. 冶金原理[M]. 北京: 科学出版社, 2005: 318-320.
[26] LI Hong-gui. Principle of metallurgy[M]. Beijing: Science Press, 2005: 318-320.
[27] 傅献彩, 沈文霞, 姚天扬. 物理化学[M]. 北京: 高等教育出版社, 1995: 725-726.
[28] FU Xian-cai, SHEN Wen-xia, YAO Tian-yang. Physical chemistry[M]. Beijing: Higher Education Press, 1995: 725-726.
(编辑 李艳红)
基金项目:广东省重大科技专项资助项目(2008 A090300016)
收稿日期:2009-12-01;修订日期:2010-03-09
通信作者:郑雅杰,教授,博士;电话:0731-88836285;E-mail:zzyyjj01@yahoo.com.cn