铝酸钠溶液中草酸钙的行为
来源期刊:中国有色金属学报(英文版)2017年第8期
论文作者:刘桂华 董文波 齐天贵 周秋生 彭志宏 李小斌
文章页码:1878 - 1887
关键词:草酸钙;铝酸钠溶液;碳酸钠;苛化;活度系数
Key words:calcium oxalate; sodium aluminate solution; sodium carbonate; lime causticisation; activity coefficient
摘 要:草酸钙的稳定性对于铝酸钠溶液中草酸钠的脱除至关重要。研究了草酸钙在含有碳酸钠的铝酸钠溶液中的反应行为。结果表明,在铝酸钠溶液和碳酸钠溶液中,草酸钙能够分别被转化为铝酸三钙(TCA)和碳酸钙。在铝酸钠溶液中,升高温度、延长反应时间以及增加苛性碱浓度会使得草酸钙的转化率增加,进而发生反苛化反应。此外,含钙化合物的稳定性在含有碳酸钠的铝酸钠溶液中与其在单一的铝酸钠或者碳酸钠溶液中不同。铝酸钠溶液中的碳酸钠会促进草酸钙的转化,因此4CaO·Al2O3·CO2·11H2O和TCA的形成会导致氧化铝的损失。在铝酸钠溶液中,温度升高会促进碳酸钙、4CaO·Al2O3·CO2·11H2O和草酸钙转化为TCA。在低温稀铝酸钠溶液中,短停留时间操作条件下,草酸钙可以保持相对稳定。研究的新型石灰苛化去除草酸钠工艺已经应用于国内某氧化铝厂。
Abstract: The stability of calcium oxalate is critical for the removal of sodium oxalate from sodium aluminate solutions. This study investigated the behavior of calcium oxalate in sodium aluminate solution containing sodium carbonate. Results show that calcium oxalate can be converted to tricalcium aluminate hydrate (TCA) and calcium carbonate in sodium aluminate solution and sodium carbonate solution, respectively. Elevating temperature, extending residence time, or increasing caustic soda concentration enhances the conversion ratio of calcium oxalate in sodium aluminate solution; as a consequence, anti-causticisation occurs. Stability of calcium-containing compounds in sodium aluminate solution containing sodium carbonate differs from that in sodium aluminate solution or sodium carbonate solution. Na2CO3 in aluminate solution accelerates the transformation of calcium oxalate; thus, alumina is lost because of 4CaO·Al2O3·CO2·11H2O and TCA formation. Calcium carbonate, 4CaO·Al2O3·CO2·11H2O and calcium oxalate can change into TCA in sodium aluminate solution at elevated temperature. Calcium oxalate remains relatively stable in dilute aluminate solution within a short residence time at low temperature. Thus, a novel process for removal of sodium oxalate by lime causticisation was presented and employed in an alumina refinery in China.
Trans. Nonferrous Met. Soc. China 27(2017) 1878-1887
Gui-hua LIU, Wen-bo DONG, Tian-gui QI, Qiu-sheng ZHOU, Zhi-hong PENG, Xiao-bin LI
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 24 December 2015; accepted 30 December 2016
Abstract: The stability of calcium oxalate is critical for the removal of sodium oxalate from sodium aluminate solutions. This study investigated the behavior of calcium oxalate in sodium aluminate solution containing sodium carbonate. Results show that calcium oxalate can be converted to tricalcium aluminate hydrate (TCA) and calcium carbonate in sodium aluminate solution and sodium carbonate solution, respectively. Elevating temperature, extending residence time, or increasing caustic soda concentration enhances the conversion ratio of calcium oxalate in sodium aluminate solution; as a consequence, anti-causticisation occurs. Stability of calcium-containing compounds in sodium aluminate solution containing sodium carbonate differs from that in sodium aluminate solution or sodium carbonate solution. Na2CO3 in aluminate solution accelerates the transformation of calcium oxalate; thus, alumina is lost because of 4CaO·Al2O3·CO2·11H2O and TCA formation. Calcium carbonate, 4CaO·Al2O3·CO2·11H2O and calcium oxalate can change into TCA in sodium aluminate solution at elevated temperature. Calcium oxalate remains relatively stable in dilute aluminate solution within a short residence time at low temperature. Thus, a novel process for removal of sodium oxalate by lime causticisation was presented and employed in an alumina refinery in China.
Key words: calcium oxalate; sodium aluminate solution; sodium carbonate; lime causticisation; activity coefficient
1 Introduction
Sodium oxalate, generated in the Bayer process by decomposition of organic compounds in bauxite, is a well-known organic impurity in sodium aluminate solution [1]. Low solubility and easy co-precipitation with alumina trihydrate (ATH) in seeded precipitation processes lead to low seed precipitation rate, fine ATH, poor alumina quality, considerable caustic soda loss, and difficult slurry filtration [2-6]. Moreover, sodium oxalate deteriorates the settlement property of red mud and promotes the formation of scales in evaporation equipment and precipitation tanks [7,8]. Sodium oxalate, together with sodium carbonate and sodium sulfate in aluminate solution, further accelerates the formation of scales on equipment surface and exacerbates seeded precipitation [9].
Sodium oxalate can be removed from solutions through various methods, including crystallization [10,11], fine ATH washing [12,13], precipitation [14], wet oxidation [15], ion exchange [16], adsorption [17], and causticisation [14,18,19]. Compared with other methods, lime causticisation is extensively applied because of several advantages, such as caustic soda recovery, convenient operation, and low cost. Thus, lime causticisation has been improved. ZHAO et al [20] proposed that lime can be added to oxalate-rich aluminate solution to form insoluble calcium oxalate. The addition of lime depends on the stoichiometric coefficients of CaO to Al2O3,  ,
,  , and
, and 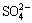 in solutions according to the common understanding that tricalcium aluminate hydrate (TCA), calcium carbonate and calcium oxalate can be formed in aluminate solutions containing sodium carbonate. To avoid the negative effect of aluminate anion and remove both sodium oxalate and sodium sulfate, ROSENBERG et al [14,21] presented a two-step causticisation process. In this process, hydrocalumite is formed in the first step and calcium oxalate is produced in the second step. In ROSENBERG’s [21] subsequent patents, the utilization ratio of lime is enhanced from 20% to 80%, and alumina loss is reduced by adding sodium carbonate to recover alumina from TCA. These studies focused on calcium oxalate formation and causticisation efficiency, while more lime is required and lower efficiency occurs in practical as comparison with the above theoretical results. Moreover, the slurry containing calcium oxalate is practically pumped into the washing tanks of red mud with a relatively high concentration solution (caustic soda ρ(Na2O) >40 g/L, alumina ρ(Al2O3) >40 g/L) for 3-10 h at 90 °C to 100 °C to simplify the treatment of causticisation slurry. An interconvertible reaction may occur among calcium-containing compounds during the washing of red mud because the solubilities of these calcium-containing compounds vary [22]. Consequently, lime causticisation efficiency is further reduced. However, the behavior of calcium oxalate in sodium aluminate solutions has been rarely reported, and the influence of sodium carbonate in aluminate solutions on causticisation remains unclear.
in solutions according to the common understanding that tricalcium aluminate hydrate (TCA), calcium carbonate and calcium oxalate can be formed in aluminate solutions containing sodium carbonate. To avoid the negative effect of aluminate anion and remove both sodium oxalate and sodium sulfate, ROSENBERG et al [14,21] presented a two-step causticisation process. In this process, hydrocalumite is formed in the first step and calcium oxalate is produced in the second step. In ROSENBERG’s [21] subsequent patents, the utilization ratio of lime is enhanced from 20% to 80%, and alumina loss is reduced by adding sodium carbonate to recover alumina from TCA. These studies focused on calcium oxalate formation and causticisation efficiency, while more lime is required and lower efficiency occurs in practical as comparison with the above theoretical results. Moreover, the slurry containing calcium oxalate is practically pumped into the washing tanks of red mud with a relatively high concentration solution (caustic soda ρ(Na2O) >40 g/L, alumina ρ(Al2O3) >40 g/L) for 3-10 h at 90 °C to 100 °C to simplify the treatment of causticisation slurry. An interconvertible reaction may occur among calcium-containing compounds during the washing of red mud because the solubilities of these calcium-containing compounds vary [22]. Consequently, lime causticisation efficiency is further reduced. However, the behavior of calcium oxalate in sodium aluminate solutions has been rarely reported, and the influence of sodium carbonate in aluminate solutions on causticisation remains unclear.
In this work, the stability of calcium oxalate in a Na2O-Al2O3-CO2-H2O system was investigated on the basis of the thermodynamic calculations and conversion ratios of calcium oxalate in different solutions. The distribution of calcium-containing compounds after calcium oxalate reacted with different solutions was also determined. The study provides a guide to efficiently remove sodium oxalate from sodium aluminate solutions and to reduce alumina loss through lime causticisation, which is proven in an alumina refinery in China.
2 Thermodynamic calculation of reaction between calcium oxalate and sodium aluminate solution or sodium carbonate solution
2.1 Relationship between temperature and Gibbs free energy of reactions
 ,
,  ,
,  , or
, or 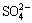 anions in sodium aluminate solution can react with calcium hydroxide to generate the corresponding calcium- containing compounds [12,14,20,23]. The following calcium-containing compounds can be formed in sodium aluminate solutions in alumina refineries without regard to CaSO4·2H2O with a relatively high solubility.
anions in sodium aluminate solution can react with calcium hydroxide to generate the corresponding calcium- containing compounds [12,14,20,23]. The following calcium-containing compounds can be formed in sodium aluminate solutions in alumina refineries without regard to CaSO4·2H2O with a relatively high solubility.
Ca(OH)2+H2O+
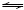 CaC2O4·H2O+2OH- (1)
CaC2O4·H2O+2OH- (1)
Ca(OH)2+2/3
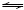 1/3(3CaO·Al2O3·6H2O)+2/3OH- (2)
1/3(3CaO·Al2O3·6H2O)+2/3OH- (2)
Ca(OH)2+1/2 +3/2H2O
+3/2H2O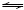 1/4(4CaO·Al2O3·13H2O)+1/2OH- (3)
1/4(4CaO·Al2O3·13H2O)+1/2OH- (3)
Ca(OH)2+
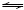 CaCO3+2OH- (4)
CaCO3+2OH- (4)
Ca(OH)2+1/2 +1/4
+1/4 +5/4H2O
+5/4H2O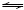 1/4(4CaO·Al2O3·CO2·11H2O)+OH- (5)
1/4(4CaO·Al2O3·CO2·11H2O)+OH- (5)
Ca(OH)2+1/2 +1/8
+1/8 +11/8H2O
+11/8H2O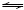 1/4(4CaO·Al2O3·1/2CO2·12H2O)+3/4OH- (6)
1/4(4CaO·Al2O3·1/2CO2·12H2O)+3/4OH- (6)
These reaction equations indicate that the increase in caustic soda concentration (OH- is expressed as Na2O) is detrimental to the stability of calcium-containing compounds. Based on the thermodynamic data [20,24-26], Gibbs free energy of reactions at different temperatures was then calculated. Results are presented in Fig. 1.

Fig. 1 Relationship between temperature and Gibbs free energy of calcium-containing compounds
Figure 1 illustrates that 4CaO·Al2O3·13H2O, CaC2O4·H2O, 3CaO·Al2O3·6H2O, CaCO3, 4CaO·Al2O3·CO2·11H2O, and 4CaO·Al2O3·1/2CO2·12H2O (CaO·
Al2O3·xCO2·12H2O is known as hydrocalumite) can be formed by adding Ca(OH)2 at temperatures below 375 K. Among these compounds, CaCO3 is the most stable and 4CaO·Al2O3·13H2O is decomposed at increased temperature. 4CaO·Al2O3·CO2·11H2O is more stable than 4CaO·Al2O3·1/2CO2·12H2O. Likewise, CaC2O4·
H2O is more stable than TCA at temperatures below 473 K. An increase in temperature promotes the transformation of 4CaO·Al2O3·1/2CO2·12H2O, 4CaO·
Al2O3·CO2·11H2O, and CaC2O4·H2O into TCA. CaC2O4·H2O is also converted to CaCO3 at temperatures above 317 K.
2.2 Stability of calcium oxalate in sodium carbonate solutions or sodium aluminate solutions
To understand the effect of sodium carbonate and aluminate anion on the stability of calcium oxalate, the behavior of calcium oxalate was discussed on the basis of the thermodynamic equilibrium calculation of the chemical reactions as Eq. (8)-(9) in the simulated oxalate-rich aluminate solution from fine ATH washing process.
CaC2O4·H2O+
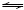 CaCO3+
CaCO3+ +H2O (7)
+H2O (7)
3CaC2O4·H2O+ +4OH-
+4OH-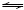 3CaO·Al2O3·6H2O +
3CaO·Al2O3·6H2O + +3H2O (8)
+3H2O (8)
Both reactions can release oxalate anion. Moreover, the occurrence of the reaction in Eq. (8) leads to alumina loss, this process is defined as anti-causticisation.
The mean activity coefficients of NaOH, NaAl(OH)4, Na2C2O4, and Na2CO3 are obtained on the basis of Bromley’s model expressed as follows [26-28]:
 (9)
(9)

 (10)
(10)
This model uses a similar expression to the FX term. In these equations, i and j represent the different ions of M and X in a given solution, respectively; Am (Debye–Hückel constant) is 0.5100 kg1/2/mol1/2 at 298.15 K [27]; and Zi and νi denote the ionic charge and stoichiometric coefficient of each ion, respectively, (ν=νM+νX); I refers to ionic strength; BMi is Bromley’s coefficient; mi is the mass molar concentration of ions (mol/kg); and BNaOH, 
 and
and 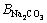 are 0.0759, 0.0188, -0.045, and 0.0001, respectively [27]. Thus, the solubility of calcium oxalate in different solutions was calculated at 298.15 K according to the following equations:
are 0.0759, 0.0188, -0.045, and 0.0001, respectively [27]. Thus, the solubility of calcium oxalate in different solutions was calculated at 298.15 K according to the following equations:
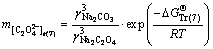 (11)
(11)

 (12)
(12)
where  and
and  refer to Gibbs free energies of Eqs. (7) and (8) at 298.15 K, respectively. R is the molar gas constant equal to 8.314 J/(mol·K). Subscripts of Tr (7) and Tr (8) refer to Eqs. (7) and (8), respectively.
refer to Gibbs free energies of Eqs. (7) and (8) at 298.15 K, respectively. R is the molar gas constant equal to 8.314 J/(mol·K). Subscripts of Tr (7) and Tr (8) refer to Eqs. (7) and (8), respectively.
Figure 2 shows the stable area of calcium- containing compounds in different solutions.
Figure 2(a) indicates that the increase in Na2CO3 concentration linearly enhances  concentration. This result suggests that a great amount of calcium oxalate is converted into calcium carbonate in the sodium carbonate solution. The stable area of CaCO3, which is located below the line in Fig. 2(a), also enlarges as the Na2CO3 concentration increases. This observation is similar to the stable area of calcium oxalate, that is, this area broadens as the sodium oxalate concentration increases. Figure 2(b) shows that the Na2C2O4 concentration increases slightly with the increase of caustic soda concentration in the solution when the ρ(Na2O) is lower than 20 g/L. While, the Na2C2O4 concentration increases significantly as the caustic soda concentration increases at ρ(Na2O) >40 g/L. This trend demonstrates that calcium oxalate is stable in the extremely dilute aluminate solution. An increase in αk enlarges the stable area of calcium oxalate; as a consequence, the decomposition of calcium oxalate in a solution containing low alumina concentration is prevented. This result also suggests that the increase in alumina concentration promotes the transformation of calcium oxalate to TCA, as presented in Eq. (8). Thus, calcium oxalate is unstable in sodium carbonate solution or the concentrated sodium aluminate solution.
concentration. This result suggests that a great amount of calcium oxalate is converted into calcium carbonate in the sodium carbonate solution. The stable area of CaCO3, which is located below the line in Fig. 2(a), also enlarges as the Na2CO3 concentration increases. This observation is similar to the stable area of calcium oxalate, that is, this area broadens as the sodium oxalate concentration increases. Figure 2(b) shows that the Na2C2O4 concentration increases slightly with the increase of caustic soda concentration in the solution when the ρ(Na2O) is lower than 20 g/L. While, the Na2C2O4 concentration increases significantly as the caustic soda concentration increases at ρ(Na2O) >40 g/L. This trend demonstrates that calcium oxalate is stable in the extremely dilute aluminate solution. An increase in αk enlarges the stable area of calcium oxalate; as a consequence, the decomposition of calcium oxalate in a solution containing low alumina concentration is prevented. This result also suggests that the increase in alumina concentration promotes the transformation of calcium oxalate to TCA, as presented in Eq. (8). Thus, calcium oxalate is unstable in sodium carbonate solution or the concentrated sodium aluminate solution.

Fig. 2 Stability of calcium oxalate in sodium carbonate (a) and sodium aluminate (b) solutions
2.3 Stability of calcium-containing compounds in sodium aluminate solutions with sodium carbonate
Sodium aluminate solutions in alumina refineries contain high concentration of sodium carbonate, which complicates the system [22]. As such, the behavior of calcium oxalate in this complex system should be urgently investigated. The alumina concentration decreases markedly in a series of washing tanks through reversal washing. To enhance our understanding of the relationship between the alumina concentration and the stability of calcium oxalate, the free caustic soda concentrations were calculated in Eqs. (1), (2), (4), and (5) at 298.15 K according to the following equations:
 (13)
(13)
 (14)
(14)
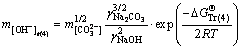 (15)
(15)

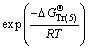 (16)
(16)
where the free caustic soda concentration m[OH-] is equal to the caustic soda concentration (caustic soda in NaOH and NaAl(OH)4) minus the caustic soda concentration in NaAl(OH)4.
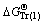 ,
, ,
,  , and
, and  represent Gibbs free energies of Eqs. (1), (2), (4), and (5) at 298.15 K, respectively.
represent Gibbs free energies of Eqs. (1), (2), (4), and (5) at 298.15 K, respectively.
The results are shown in Fig. 3.

Fig. 3 Influence of alumina concentration on stability of calcium-containing compounds
The stable area of calcium-containing compounds is located below the corresponding line; the unstable area is located above the corresponding line because ρ(OH-)>ρ(OH-)eq.
The stable area of calcium-containing compounds: 1) TCA, CaC2O4·H2O, 4CaO·Al2O3·CO2·11H2O, and CaCO3; 2) CaC2O4·H2O, 4CaO·Al2O3·CO2·11H2O, and CaCO3; 3) 4CaO·Al2O3·CO2·11H2O and CaCO3; 4) CaCO3; 5) TCA, 4CaO·Al2O3·CO2·11H2O, and CaCO3; 6) 4CaO·Al2O3·CO2·11H2O and TCA; 7) TCA; and 8) TCA and CaCO3.
In terms of activity coefficient, the increase in the Al2O3 concentration almost linearly increases OH- equilibrium concentration for each calcium-containing compound (Fig. 3). Figure 3 shows that calcium- containing compounds can co-exist in dilute solutions with low caustic soda concentration (area 1). On the basis of the upper left area above the line in Fig. 3, we can observe that calcium oxalate is more stable than TCA at an alumina concentration of ρ(Al2O3) <25 g/L (area 2), and calcium carbonate is a relatively stable substance at different concentrations of alumina and caustic soda (areas 1, 2, 3, 4, 5, and 8). Calcium oxalate can be converted to 4CaO·Al2O3·CO2·11H2O, TCA, and calcium carbonate (areas 3 and 4). In addition, calcium oxalate, 4CaO·Al2O3·CO2·11H2O, and calcium carbonate are converted to TCA as the alumina concentration increases (areas 6 and 7). Figure 3 also illustrates that the increase in the free caustic soda concentration can largely reduce the stability of hydrocalumite as the Al2O3 concentration remains constant. However, TCA is the most stable substance, but calcium oxalate is preferentially decomposed in a solution with high concentrations of caustic soda and alumina (ρ(Al2O3) >30 g/L). CaCO3 can be converted to 4CaO·Al2O3·CO2·11H2O and TCA as the alumina concentration increases. These findings are remarkably different from that shown in Fig. 1. Therefore, the behavior of calcium-containing compounds in the complex system differs from that in the single system of sodium carbonate or sodium aluminate. In addition, lime causticisation should not be performed in concentrated sodium aluminate solutions unless calcium oxalate is converted to other calcium-containing compounds.
3 Experimental
3.1 Materials
Sodium aluminate solution was prepared by industrial aluminum hydroxide (Chalco) and sodium hydroxide based on αk (1.4-3.0). CaC2O4·H2O, Na2CO3, and other reagents were of analytical grade.
3.2 Experimental procedure
Experiments were carried out in a 150 mL continuously rotating steel bomb immersed in glycerol and heated by electricity (±1 °C). Different solutions (sodium aluminate solution, sodium carbonate solution, or sodium aluminate solution with sodium carbonate) and calcium oxalate were added to the steel bomb. After the reaction was completed at a given temperature and duration, the slurry was filtered. The resulting solution was then obtained by diluting the filtrate with distilled water to produce the final of 500 mL to determine the concentrations of Al2O3 and  . The dried sludge was used to identify the contents of calcium-bearing compounds.
. The dried sludge was used to identify the contents of calcium-bearing compounds.
3.3 Characterization
The alumina (Al2O3) and caustic soda (Na2O) concentrations in the resulting solution were determined through titration. The sodium oxalate (Na2C2O4) concentration was obtained by using an ICS-90A ion chromatograph (DIONEX) [29]. The X-ray diffraction patterns of the sample were obtained by using a powder diffractometer (D8-Advance, Bruker Corporation). The elemental distribution of the sludge was observed with an energy dispersive spectrometer (EDS, JSM-6360LV, Electronic, Ltd.).
3.4 Data processing
The conversion ratio of calcium oxalate in either sodium carbonate solution or sodium aluminate solution was calculated according to the following formula:
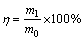 (17)
(17)
where m0 and m1 correspond to  contents in calcium oxalate and in solution (g), respectively.
contents in calcium oxalate and in solution (g), respectively.
The mass fraction of the corresponding calcium- containing compound (taking CaCO3 for example) in sludge (ω) was calculated using the following formula, with the total calcium ([Ca]T) content remaining constant:
 (18)
(18)
where subscript T refers to the total calcium content and the other subscripts denote the contents of the corresponding calcium-containing compounds.
The CaC2O4·H2O and CaCO3 contents in the sludge were obtained on the basis of the concentration variation of oxalate anion and carbonate anion in the solution; the TCA and 4CaO·Al2O3·CO2·11H2O contents were calculated according to the total mass of the sludge and concentration variation of alumina before and after reaction. The mass fraction of the calcium-containing compounds was then determined.
4 Results and discussion
4.1 Influence of temperature on reaction between calcium oxalate and different solutions
In practice, causticisation temperature varies in causticisation-related processes, including lime causticisation reaction, clarification, and washing operation. In the aluminate solution containing sodium carbonate, calcium oxalate may react with sodium carbonate (expressed as Na2Oc) or sodium aluminate solution, as expressed in Eqs. (7) and (8). Figure 4 illustrates the variation in the conversion ratio of calcium oxalate in the aluminate solution or the carbonate solution to describe the influence of reaction temperature on the stability of calcium oxalate in the aluminate solution.

Fig. 4 Influence of temperature on conversion ratio of calcium oxalate
Figure 4 shows that increasing temperature favors the increase in the conversion ratio of calcium oxalate, which illustrates that increasing temperature accelerates the transformation of calcium oxalate into calcium carbonate and TCA in the sodium carbonate solution and in the sodium aluminate solution, respectively. The conversion ratio of calcium oxalate increases remarkably in the sodium carbonate solution as temperature increases because calcium carbonate is preferentially formed at high temperature (Fig. 1). By contrast, the conversion ratio of calcium oxalate almost remains constant at temperatures of <330 K and then increases significantly with temperature rising in sodium aluminate solution. This finding indicates that an increase in temperature causes significant anti-causticisation; the calcium oxalate-containing slurry should not return to the aluminate solution at high temperature.
4.2 Influence of residence time on reaction between calcium oxalate and aluminate solution
The slurry or the concentrated slurry (underflow) after settling is practically pumped to the second washing tank (ρ(Na2O) 30-45 g/L) to simplify the process. The slurry then flows through the third tank, fourth tank and the final washing tank for 5-8 h at 90-95 °C. Thus, residence time is a critical factor to ensure sufficient causticisation. The influence of residence time on the conversion ratio of calcium oxalate in the sodium aluminate solution is presented in Fig. 5.

Fig. 5 Influence of residence time on conversion ratio of calcium oxalate (a) and mass distribution of calcium oxalate and TCA (b)
Figure 5(a) indicates that the conversion ratio of calcium oxalate remarkably increases with residence time in the sodium aluminate solution because of the transformation of calcium oxalate into TCA. Figure 5(b) shows that the content of calcium oxalate in the sludge decreases but the content of TCA increases with time; as a consequence, alumina is lost. For example, up to 44.75% TCA in the sludge in the first 30 min demonstrates that anti-causticisation readily occurs in the aluminate solution. Figure 5 also illustrates that calcium oxalate must be discharged alone to prevent sodium oxalate from returning to the sodium aluminate solution when anti-causticisation occurs.
To further investigate the distribution of calcium- containing compounds in the sludge after causticisation occurs, the EDS element maps (Fig. 6) are presented. The highlighted areas in Figs. 6(b) and 6(c) are the main distribution areas of Ca and Al elements, respectively.

Fig. 6 EDS element maps of sludge after calcium oxalate reacts with sodium aluminate solution
In Fig. 6, TCA and calcium oxalate are presented as fine particles size (<5 μm). Calcium oxalate is difficult to distinguish from TCA on the basis of morphological characteristics. Comparing Fig. 6(b) with Fig. 6(c), TCA in the form of element Al is scattered on the surface of calcium oxalate. TCA also co-exists with calcium oxalate, as shown in Fig. 6(b). These findings demonstrate that calcium oxalate is readily converted into TCA at temperatures higher than 90 °C.
4.3 Influence of caustic soda concentration on reaction of calcium oxalate with aluminate solution
The concentration of caustic soda in a series of washing tanks sharply decreases during the red mud washing process. Figure 7 displays the influence of caustic soda concentration on the calcium oxalate conversion ratio, alumina reaction ratio and the mass fraction of calcium-containing compounds in the sludge.

Fig. 7 Influence of caustic soda concentration on conversion ratio of calcium oxalate (a) and mass distribution of calcium oxalate and TCA (b)
Figure 7 shows that the increase in the Na2O concentration significantly enhances the conversion ratio of calcium oxalate and the reaction ratio of alumina in the sodium aluminate solution at 96 °C. The conversion ratio of calcium oxalate remains less than 10%, and the mass fraction of TCA in the sludge is less than 27% in solution of ρ(Na2O)<20 g/L. And then the conversion ratio of calcium oxalate and the mass fraction of TCA in the sludge significantly increase as the caustic soda concentration increases. Therefore, the conversion of calcium oxalate into TCA can be inhibited by reducing the concentration of caustic soda in the sodium aluminate solution. The fact suggests that calcium oxalate should be discharged alone into red mud stock dump rather than currently pumped into the first or the second red mud washing tanks to match the caustic soda concentration.
The XRD patterns of the sludge after calcium oxalate reacting with the sodium aluminate solution at 96 °C are illustrated in Fig. 8.

Fig. 8 XRD patterns of sludge after calcium oxalate reacts with sodium aluminate solution (Sodium aluminate solution
Many well-characteristic peaks in Fig. 8(a) are assigned to CaC2O4·H2O, and a few weak peaks of TCA appear in the sodium aluminate solution of ρ(Na2O)=10 g/L. However, a number of peaks of TCA are also found in the sodium aluminate solution of ρ(Na2O)=40 g/L (Fig. 8(b)). These results confirm that calcium oxalate is readily converted into TCA in the concentrated aluminate solution; as a result, anti-causticisation occurs.
4.4 Influence of sodium carbonate in sodium aluminate solution on reaction of calcium oxalate with aluminate solution containing carbonate anion
Sodium carbonate is a harmful impurity in Bayer liquor [22] and is predominantly produced from the reaction of CaCO3 with NaOH at high temperature, as expressed in the following reaction:
CaCO3+2NaOH=Ca(OH)2+Na2CO3 (19)
The pregnant sodium aluminate solution in alumina refineries often contains 5-35 g/L Na2CO3 (equal to 2.9-20.5 g/L Na2Oc). Figure 4 indicates that a part of calcium oxalate changes into calcium carbonate and TCA in the sodium carbonate solution and the sodium aluminate solution, respectively. Thus, the influence of sodium carbonate on the behavior of calcium oxalate in the sodium aluminate solution should be elucidated. Results are shown in Fig. 9.

Fig. 9 Influence of sodium carbonate in sodium aluminate solution on the conversion ratio of calcium oxalate (a) and the mass distribution of calcium-containing compounds (b)
Figure 9(a) shows that calcium oxalate readily reacts with solution within 30 min, and the conversion ratio of calcium oxalate slightly increases and then slowly reduces from 30 to 180 min. Figure 9(a) also reveals that the 69.45% conversion ratio of calcium oxalate at 60 min differs from the 45.99% conversion ratio shown in Fig. 5. This difference indicates that sodium carbonate accelerates the transformation of calcium oxalate at 96 °C because of the simultaneous reaction, as expressed in Eqs. (7) and (8). Thus, the remarkable anti-causticisation in Chinese alumina refineries accounts for a large amount of sodium carbonate generated at increased temperature (Eq. (9)). Figure 9(b) shows that the calcium oxalate content sharply reduces at 30 min and then remains unchanged as the reaction time is extended. The calcium carbonate content (<10%) nearly remains constant. The TCA content consistently increases while the hydrocalmite content increases initially and then decreases gradually with residence time. These results indicate that hydrocalmite can change into TCA, and this finding is consistent with that illustrated in Figs. 1 and 3 (area 7). The calcium carbonate content is considerably less than the TCA and hydrocalmite contents because TCA is more stable than CaCO3 in the sodium aluminate solution, as described in Fig. 3 (areas 6 and 7). Compared with Fig. 5, Fig. 9 demonstrates that calcium oxalate reacts more rapidly with solution; as a result, anti-causticisation and alumina loss occur.
The XRD patterns of the sludge are shown in Fig. 10.

Fig. 10 XRD patterns of sample after calcium oxalate reacts with sodium aluminate solution containing sodium carbonate
Calcium oxalate (CaC2O4·H2O), hydrocalmite (4CaO·Al2O3·CO2·11H2O), and calcium carbonate can be observed at 30 min (Fig. 10). The characteristic peak of TCA can be detected at 90 min, and the peak intensity increases gradually. This trend suggests that hydrocalmite is readily formed and subsequently converted to TCA. Calcium oxalate can also be converted to 4CaO·Al2O3·CO2·11H2O instead of 4CaO·Al2O3·1/2CO2·12H2O [25] in the sodium aluminate solution containing sodium carbonate. Thus, 4CaO·Al2O3·CO2·11H2O is likely formed, as illustrated in Fig. 1.
On the basis of these findings, the following conclusions can be made. Calcium oxalate can be relatively stable in dilute aluminate solution (ρ(Na2O) and ρ(Al2O3) are preferably under 20 g/L) for a very short time at low temperatures. In an alumina refinery in Shangdong Province in China, a novel process (as shown in Fig. 11) was presented to remove sodium oxalate by lime causticisation [30]. In this process, lime was added according to the molar ratio of n(CaO)/n(Na2C2O4)= 1.0-1.5 and more than 90% Na2C2O4 changed into CaC2O4·H2O. The formation of TCA and hydrocalmite was successfully inhibited in the diluted sodium aluminate solution. Moreover, as compared with the traditional lime causticisation, solid content in slurry then reduced significantly, leading to a better clarification by settlement for slurry after lime causticisation. Afterwards, the overflow was pumped into the third washing tank of red mud after the slurry well settled, the underflow was then pumped into the final washing tanks with ρ(Na2O) of 5 g/L and a residence time less than 2 h. As a result, the anti-causticisation of calcium oxalate was perfectly prevented the novel process reduces alumina loss by about 50% and saves around 60% lime. By contrast, the concentrated slurry in traditional lime causticisation is pumped into the second washing tank due to bad settlement property. This procedure causes significant anti-causticisation, which leads to large alumina loss and much sodium oxalate returning into aluminate solution.
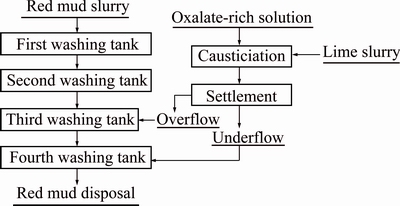
Fig. 11 Novel lime-causticisation flowsheet for removal of sodium oxalate
5 Conclusions
1) The increase in the concentration of sodium carbonate or caustic soda enlarges the stable area of calcium carbonate or TCA, respectively. Calcium oxalate is stable in dilute solutions at low temperatures; otherwise, calcium oxalate changes into 4CaO·Al2O3·1/2CO2·11H2O, 4CaO·Al2O3·CO2·11H2O, CaCO3, or TCA in aluminate solutions. The increase in the alumina concentration induces the conversion of calcium oxalate and CaCO3 to 4CaO·Al2O3·CO2·11H2O and TCA. 4CaO·Al2O3·CO2·11H2O is a more stable substance than 4CaO·Al2O3·1/2CO2·11H2O.
2) Calcium oxalate can be converted to TCA and calcium carbonate in sodium aluminate solution and sodium carbonate solution, respectively. The conversion ratio of calcium oxalate in the sodium aluminate solution and anti-causticisation increase with temperature, residence time, and caustic soda concentration.
3) Sodium carbonate in sodium aluminate solution complicates the reaction behavior of calcium-containing compounds; sodium carbonate in aluminated solution favors the formation of 4CaO·Al2O3·CO2·11H2O and TCA instead of calcium carbonate. Sodium carbonate significantly increases the conversion ratio of calcium oxalate and reduces the content of calcium oxalate in sludge. 4CaO·Al2O3·CO2·11H2O can be converted to TCA in the sodium aluminate solution at increased temperature. The formation of hydrocalmite and TCA causes alumina loss.
4) Anti-causticisation is inhibited when a novel process of lime causticisation was adopted in an alumina refinery. Compared with the traditional process, the novel process reduced about 50% alumina loss and saved around 60% lime addition.
References
[1] KIM M J, LEE S O. Overview of the behaviour of sodium oxalate in Bayer liquor and its effect of the process [C]//Light Metals. San Diego, California: TMS, 2003: 19-24.
[2] BUSETTI F, BERWICK L, MCDONALD S, HEITZ A, JOLL C A, LOH J, POWER G. Physicochemical characterization of organic matters in Bayer liquor [J]. Industrial & Engineering Chemistry Research, 2014, 53(15): 6544-6553.
[3] POWER G, LOH J. Organic compounds in the processing of lateritic bauxites to alumina. Part 1: Origins and chemistry of organics in the Bayer process [J]. Hydrometallurgy, 2010, 105(1): 1-29.
[4] BROWN N. Method for removing sodium oxalate from caustic aluminate liquors: US, US4999170[P]. 1991-03-12.
[5] BROWN N, COLE T J. The behaviour of sodium oxalate in a Bayer alumina plant [C]//Light Metals. Las Vegas, Nevada: TMS, 1980: 105-117.
[6] POWER G, LOH J, VERNON C. Organic compounds in the processing of lateritic bauxites to alumina. Part 2: Effects of organics in the Bayer process [J]. Hydrometallurgy, 2012, 127(18): 125-149.
[7] SMEULDERS D E, WILSON M A, ARMSTRONG L. Insoluble organic compounds in the Bayer process [J]. Industrial & Engineering Chemistry Research, 2001, 40(10): 2243-2251.
[8] POWER G, LOH J S C, WAJON J E, BUSETTI F, JOLL C. A review of the determination of organic compounds in Bayer process liquors [J]. Analytica Chimica Acta, 2011, 689(1): 8-21.
[9] MAHMOUDIAN M, GHAEMI A, SHAHHOSSEINI S. Removal of carbonate and oxalate pollutants in the Bayer process using thermal and chemical techniques [J]. Hydrometallurgy, 2015,154(3): 137-148.
[10] MCCAUSLAND L J, FENNELL M. Removal of sodium oxalate from a bayer liquor: CA, CA2556665A1[P]. 2009-04-02.
[11] FABRE J, LAVALOU E, NICOLAS F. Purification of solutions of sodium aluminate in the Bayer cycle by the removal of sodium oxalate: US, US4597952A[P]. 1986-07-01.
[12] ROSENBERG S P, TICHBON W, HEALY S J. Organic impurity removal process for Bayer liquors: US, US6555077[P]. 2003-04-29.
[13] TAYLOR M, HARRIS D J, CHEN H T, COCALIA V. Methods and compositions for the removal of impurities and water from the Bayer process: US, US7972580[P]. 2011-07-05.
[14] ROSENBERG S P, TICHBON W, WILSON D J, HEATH C A. Process for the removal of oxalate and /or sulphate from Bayer liquors: US, US6743403[P]. 2004-06-01.
[15] DONALDSON D, RAAHAUGE B E. Removal of organic carbon from bayer liquor by wet oxidation in tube digesters [C]//Light Metals. Warrendale, Pennsylvania: TMS, 2013: 304-308.
[16] HIND A R, BHARGAVA S K, GROCOTT S C. The surface chemistry of Bayer process solids: A review [J]. Colloids Surface A Physicochemical & Engineering Aspects, 1999, 146(1): 359-374.
[17] WILLIAMS S F. Adsorbent combinations for enhanced removal of sodium oxalate from Bayer process spent liquor: US, US5728180A [P]. 1998-03-17.
[18] SOIRAT A. Process for purifying sodium aluminate solutions containing sodium oxalate: US, US5888461 [P]. 1999-03-30.
[19] SATO C, FURUKAWA A, SANO Y. Method for removing impurities in the Bayer process: US, US3649185[P]. 1972-03-14.
[20] ZHAO Yu, LIU Gui-hua, PI Jian-qing. Lime causticisation of sodium oxalate in sodium aluminate solution [J]. World Nonferrous Metal, 2013, 11: 76-77. (in Chinese)
[21] ROSENBERG S P, WILSON D J, HEATH C A. Inhibiting the formation of TCA in a Bayer causticisation process: US Patent, 7767190[P]. 2010-08-03.
[22] PERROTTA A J, WILLIAMS F. Hydrocalumite formation in Bayer liquor and its promotional effect on oxalate precipitation [C]//Light Metals. Warrendale, Pennsylvania: TMS, 1995: 77-87.
[23] WELLINGTON M, VALCIN F. Impact of bayer process liquor impurities on causticization [J]. Industrial & Engineering Chemistry Research, 2007, 46(15): 5094-5099.
[24] MATSCHEI T, LOTHENBACH B, GLASSER F P. Thermodynamic properties of Portland cement hydrates in the system CaO-Al2O3- SiO2-CaSO4-CaCO3–H2O [J]. Cement & Concrete Research, 2007, 37(10): 1379-1410.
[25] BUTTERY J H N, PATRICK V A, ROSENBERG S P, HEATH C A, WILSON D J. Thermodynamics of hydrocalumite formation in causticisation [C]//Light Metals. Settle, Washington: TMS, 2002: 185-192.
[26] RAPOSO J C, SANZ J, BORGE G, OLAZABAL M A, MADARIAGA J M. Development of a Modified Bromley’s methodology for the estimation of ionic media effects on solution equilibria. Part 3: Application to the construction of thermodynamic models [J]. Fluid Phase Equilibria, 1999, 155(155): 1-19.
[27] PENG Xiao-qi, SONG Guo-hui, SONG Yan-po, ZHANG Jian-zhi, Liu Zhen-guo. Calculation model of activity coefficient for NaOH- NaAl(OH)4-Na2CO3-H2O system [J]. Chinese Journal of Nonferrous Metals, 2009, 19(7): 1332-1337. (in Chinese)
[28] LI Xiao-bin,  Wei-jun, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng, MENG Yun, REN Wan-neng. Activity coefficient calculation model for NaAl(OH)4-NaOH-H2O system [J]. Journal of Central South University, 2005, 37(4): 908-912.
Wei-jun, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng, MENG Yun, REN Wan-neng. Activity coefficient calculation model for NaAl(OH)4-NaOH-H2O system [J]. Journal of Central South University, 2005, 37(4): 908-912.
[29] BARNETT N W, BOWSER T A, RUSSELL R A. Determination of oxalate in alumina process liquors by ion chromatography with post column chemiluminescence detection [J]. Analytical Proceedings, 1995, 32(2): 57-59.
[30] PI Jian-qing, ZHAO Yu, LIU Gui-hua, WANG Yong-lin. A method to remove sodium oxalate from sodium aluminate solution by adding lime: CN, CN103342377A[P]. 2013-10-09.
刘桂华,董文波,齐天贵,周秋生,彭志宏,李小斌
中南大学 冶金与环境学院,长沙 410083
摘 要:草酸钙的稳定性对于铝酸钠溶液中草酸钠的脱除至关重要。研究了草酸钙在含有碳酸钠的铝酸钠溶液中的反应行为。结果表明,在铝酸钠溶液和碳酸钠溶液中,草酸钙能够分别被转化为铝酸三钙(TCA)和碳酸钙。在铝酸钠溶液中,升高温度、延长反应时间以及增加苛性碱浓度会使得草酸钙的转化率增加,进而发生反苛化反应。此外,含钙化合物的稳定性在含有碳酸钠的铝酸钠溶液中与其在单一的铝酸钠或者碳酸钠溶液中不同。铝酸钠溶液中的碳酸钠会促进草酸钙的转化,因此4CaO·Al2O3·CO2·11H2O和TCA的形成会导致氧化铝的损失。在铝酸钠溶液中,温度升高会促进碳酸钙、4CaO·Al2O3·CO2·11H2O和草酸钙转化为TCA。在低温稀铝酸钠溶液中,短停留时间操作条件下,草酸钙可以保持相对稳定。研究的新型石灰苛化去除草酸钠工艺已经应用于国内某氧化铝厂。
关键词:草酸钙;铝酸钠溶液;碳酸钠;苛化;活度系数
(Edited by Yun-bin HE)
Foundation item: Project (51274242) supported by the National Natural Science Foundation of China; Project (2015CX001) supported by the Innovation- driven Plan of Central South University, China
Corresponding author: Tian-gui QI; Tel/Fax: +86-731-88830453; E-mail: qitiangui@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60212-7

