文章编号:1004-0609(2010)09-1822-07
铬铁矿氧化焙烧动力学
李小斌, 齐天贵, 彭志宏, 刘桂华, 周秋生
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:用于铬盐生产的传统无钙焙烧过程存在铬氧化率低的问题,严重影响铬的生产效率和回收率。为强化铬铁矿氧化焙烧过程,对铬氧化过程的动力学规律及其氧化反应机理进行了研究。结果表明:铬铁矿氧化焙烧过程,铬的氧化反应分为初期和后期两个阶段,初期铬氧化速率快,后期铬氧化速率慢,在优化条件下,铬(Ⅲ)的氧化率可达99%以上;两个阶段铬的氧化反应均符合收缩未反应核动力学模型且受表面化学反应控制,铬氧化反应初期和后期的表观反应活化能分别为68.7和231.8 kJ/mol;反应初期是铬铁矿直接与碳酸钠和氧气发生反应生成铬酸钠,而后期则是铬铁矿与反应初期生成的铁酸钠和氧气发生反应生成铬酸钠。
关键词:铬铁矿;铬酸钠;氧化焙烧;动力学
中图分类号:TF111 文献标志码:A
Kinetics of chromite ore in oxidation roasting process
LI Xiao-bin, QI Tian-gui, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The traditional lime-free roasting process used for manufacturing chromate from chromite ore has low oxidation rate which impact the chromate production efficiency and chromium recovery rate seriously. To intensify the oxidation roasting process of chromite ore, the oxidation kinetics and its mechanism were studied. The results show that there are two stages for the oxidation roasting process of chromite ore. The oxidation reaction is very rapid in the initial stage but quite slow in the later stage. The oxidation rate of chromium(Ⅲ) in chromite ore can reach more than 99% under the optimized conditions. The whole oxidation process of chromite ore follows the shrinking unreacted core model, and the surface chemical reaction is the controlling step. The apparent activation energies of the initial and later stages are 68.7 and 231.8 kJ/mol, respectively. And the sodium chromate is produced by the reaction of chromite with sodium carbonate and oxygen in the initial stage, whereas sodium chromate is formed by the reaction of chromite with sodium ferrite formed in the initial stage and oxygen in the latter stage.
Key words: chromite ore; sodium chromate; oxidation roasting; kinetics
铬铁矿氧化焙烧法几乎是铬盐生产的唯一方法,其中分为有钙焙烧法和无钙焙烧法,它们都是将铬铁矿与纯碱在高温氧化条件下进行焙烧,使矿石中的铬转化为水溶性的铬酸钠经浸出等后续处理获得铬盐产 品[1-3]。有钙焙烧法由于在焙烧炉料中加入了大量的含钙填料,生产过程铬回收率低,铬渣排放量大且含有可致癌的高毒性铬酸钙,环境污染严重[4-5];无钙焙烧法使用返渣代替含钙填料,铬渣排放量大大降低且不含高毒铬酸钙,逐步成为铬盐生产的主要方法。但在无钙焙烧过程中,铬氧化速度慢、炉内铬的转化率仅75%左右,使得大量返渣在焙烧系统循环,严重影响铬的生产效率和回收率,铬污染问题也未得到彻底解决[6-7]。
为解决无钙焙烧过程存在的问题,人们对铬铁矿焙烧过程进行了大量研究,但对于铬铁矿的氧化机理至今仍未形成统一的认识。ANTONY等[8]通过研究不同气氛下铬铁矿的焙烧过程认为,当焙烧温度较低时,亚铬酸钠可能是铬铁矿氧化焙烧过程的中间体,而在较高温度下铬铁矿可直接生成铬酸钠。纪柱等[9,10]通过研究不同配碱量条件下铬铁矿氧化焙烧过程物相的变化,提出铬铁矿氧化时,铬铁尖晶石中的亚铁首先被氧化成赤铁矿,进而尖晶石中的Cr3+被氧化脱离晶格形成铬酸钠。他认为在现行无钙焙烧条件下,有少部分碳酸钠生成硅酸钠、铝酸钠等,而大部分碳酸钠直接同铬铁矿反应生成铬酸钠。VILAS等[11]认为铬铁矿氧化焙烧过程形成的Na2CrO4-Na2CO3低共熔体对铬氧化机理会产生重要影响,在较高的焙烧温度下,Na2CrO4-Na2CO3低共熔体严重阻碍铬铁矿的氧化反应,而氧气在共熔体中的扩散是铬铁矿氧化焙烧后期反应的控制步骤。
基于以上铬铁矿氧化焙烧机理,人们曾提出了铬铁矿两段焙烧法[12]、球团焙烧法[13]等强化铬铁矿氧化焙烧的方法,但由于对铬氧化反应机理缺乏明确而统一的认识,这些方法都没有真正解决铬铁矿氧化焙烧过程存在的问题。为强化铬铁矿的氧化焙烧过程,本文作者前期对铬铁矿的氧化焙烧过程进行了系统的研究,由此提出了采用添加剂强化铬铁矿氧化焙烧的技术[14],在较为宽松的条件下使铬矿物几乎完全氧化。因此,本文作者对此过程中铬的氧化反应动力学和反应机理进行研究,以期为铬盐生产过程提供理论指导。
1 实验
1.1 实验原料
铬铁矿取自国内某铬盐厂,主要矿物组成为铬镁(铁)尖晶石和少量橄榄石(见图1),铬铁矿的主要化学组成列于表1,实验用碳酸钠为分析纯试剂。
表1 铬铁矿的化学组成
Table 1 Chemical composition of chromite ore (mass fraction, %)

1.2 实验设备
实验所用设备和仪器如下:多功能矿物混合机,

图1 铬铁矿的XRD谱
Fig.1 XRD pattern of chromite ore
长沙索拓科学仪器设备有限公司;SX2-5-12型马弗炉,长沙华新合金机电有限公司;SHY-14-13型管式炉,长沙中华电炉厂;V-0.12/8型空气压缩机,天津空气压缩机有限公司;STA 409 PC型同步热分析仪,德国耐驰仪器制造有限公司。
1.3 实验方法
铬铁矿和碳酸钠分别用振动磨磨细至全部过孔径为62 um的分样筛,再用孔径为45 um的分样筛筛分磨细后的物料,使实验用铬铁矿和碳酸钠的粒度均在45~62 μm之间。将磨细后的铬铁矿、碳酸钠和添加剂按一定比例称量,装入混料瓶中,在对辊式矿物混料机上混合2 h,使物料混合均匀;混合物料用水喷雾造球,球团直径约4~6 mm,将球团在300 ℃的马弗炉中烘干1 h后得到干球团物料。每次取10 g干球团物料放入条形坩埚中,放入一定温度的管式气氛炉内,通入压缩空气进行氧化焙烧;焙烧熟料磨细混匀后,采用硫酸亚铁铵容量法分析熟料中的六价铬和总铬的含量,计算铬的氧化率,利用EPD1700型X射线衍射仪分析熟料物相。
2 结果与讨论
2.1 焙烧温度和时间对铬氧化率的影响
为研究铬铁矿氧化焙烧动力学,对不同焙烧温度和时间下,铬铁矿中铬氧化率的变化规律进行了实验研究,其实验结果如图2所示。
由图2可以看出,温度对铬氧化率的影响较大,温度越高,铬氧化速率越快、氧化率越高。在研究温

图2 不同焙烧温度和时间对铬氧化率的影响
Fig.2 Effects of roasting temperature and duration time on oxidation rate of chromium(Ⅲ) in chromite ore
度范围内(1 223~1 373 K),铬铁矿中的铬在0~20 min内的氧化速率很快,而后期反应速率较慢,如在1 323 K下,铬在0~20 min的氧化率达到90.34%,而20~100 min的氧化率仅增加9.15%,即铬的氧化过程可分为初期的快速氧化阶段和后期的缓慢氧化阶段。
2.2 铬氧化反应动力学
铬铁矿氧化焙烧过程可看作致密固体铬铁矿和碳酸钠与空气中氧气之间进行的气-固反应,高温条件下反应生成的铬酸钠可能包裹在铬铁矿与碳酸钠混合物料的表面,阻碍氧气的扩散。因此,铬铁矿氧化焙烧过程的反应符合有产物层生成的收缩未反应核动力学模型。
根据收缩未反应核模型,铬铁矿的氧化过程需经过以下步骤:O2传质通过固体表面边界层的外扩散步骤,O2扩散通过铬酸钠等生成物层到达反应界面的内扩散步骤和O2与铬铁矿等发生氧化反应的化学反应步骤,其中速度最慢的步骤将成为铬氧化过程的控制步骤。若假设铬铁矿为粒度和表面化学活性均一的球形颗粒,且在氧化过程中固态试样的体积变化不大,根据气-固反应的收缩未反应核动力学模型,反应受外扩散、内扩散和化学反应控制时,铬的氧化率x与氧化焙烧时间t之间应分别满足式(1)、(2)和(3)所示的特征线性函数关系,根据实验数据是否满足这些线性关系可判断反应的控制步骤[15]。为明确铬氧化过程的控制步骤,将1323K下铬铁矿氧化焙烧过程中铬的氧化率分别代入上述3个公式的左端并对时间作图,结果如图3所示。
x=kt (1)
1+2(1-x)-3(1-x)2/3=kt (2)
1-(1-x)1/3=kt (3)
由图3(a)可以看出,铬铁矿氧化焙烧过程铬的氧
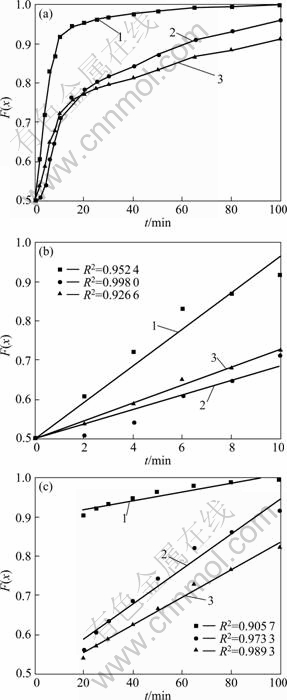
图3 1323K下不同动力学控制模型函数与时间之间的关系
Fig. 3 Relationships between different model functions and oxidation time at 1 323 K: (a) Whole process; (b) Initial stage; (c) Later stage. Curve 1—F(x)=x; Curve 2—F(x)=1+2(1-x) -3(1-x)2/3, Curve 3—F(x)=1- (1-x)1/3
化率按照收缩未反应核模型进行数据处理时,各种控制模型的特征函数与焙烧时间之间都不呈现单一的线性关系,它们均在反应时间为15~20 min范围内发生偏移,使特征函数与时间之间的关系曲线分为反应初期和反应后期两段。这说明随着铬铁矿氧化过程的进行,反应的控制步骤或反应机理发生了变化。
为明晰铬铁矿氧化过程的动力学规律,将1 323 K下铬铁矿氧化反应初期(0~10 min)和后期(20~100 min)的铬氧化率数据分别带入各控制模型的特征函数并利用最小二乘法将特征函数对焙烧时间进行线性回归分析,数据处理结果如图3(b)和(c)所示。由图3(b)和(c)可以看出,铬氧化反应初期和后期的动力学数据均符合化学反应控制模型,其特征函数F(x)=1- (1-x)1/3与时间t之间线性相关系数最大,这说明在1 323 K温度下,铬铁矿氧化过程初期和后期均受化学反应控制。为得到各阶段铬氧化的动力学参数,分析铬氧化反应过程机理,分别将不同温度下氧化反应初期和后期铬氧化率带入化学反应控制模型的特征函数F(x)=1- (1-x)1/3,并将F(x)对时间t作图,结果如图4所示。

图4 不同温度下1- (1-x)1/3与时间的关系
Fig. 4 Relationship between 1- (1-x)1/3 and time at different roasting temperatures: (a) Initial stage; (b) Later stage
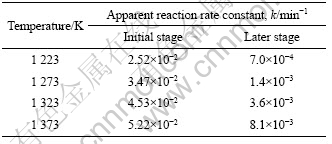
由图4可知,在1 223~1 373 K的温度范围内,铬铁矿氧化焙烧初期和后期铬的氧化率和焙烧时间之间分别符合式(3)所示的直线关系,说明在该温度范围内,铬氧化过程均符合收缩未反应核动力学的化学反应控制模型。根据直线的斜率可以求出各焙烧温度下氧化反应初期和后期的表观反应速率常数k,如表2所列。由表2中数据可以看出,反应初期的表观速率常数比后期的大,二者相差一个数量级,这说明在研究温度范围内,随着反应的进行铬的氧化反应机理发生了变化。
表2 不同温度下铬氧化反应前期和后期的表观反应速率常数k
Table 2 Apparent reaction rate constant of chromium oxidation at different roasting temperatures
根据不同温度下得到的铬氧化反应初期和后期的表观速率常数,可由阿伦尼乌斯公式计算各反应阶段的表观反应活化能,即
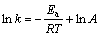 (4)
(4)
式中:k为表观反应速率常数;Ea为表观反应活化能,J/mol;R为摩尔气体常数,R=8.314 J/mol;T为绝对温度,K;A为指前因子。
将不同焙烧温度下铬氧化反应初期和后期的反应速率常数分别以lnk对1/T作图,结果如图5所示。由图中直线的斜率可求出氧化反应初期和后期的表观反应活化能分别为68.7和231.8 kJ/mol,这进一步证明铬的氧化反应过程受化学反应控制,且反应初期和后期的反应机理不同。
2.3 铬氧化反应机理分析
铬铁矿氧化焙烧过程中,铬铁尖晶石与碳酸钠和氧气之间可能的主要反应如下:
FeCr2O4+2Na2CO3+7/4O2=2Na2CrO4+1/2Fe2O3+2CO2
(5)
FeCr2O4+Na2CO3+1/4O2=Na2Cr2O4+1/2Fe2O3+CO2
(6)
Fe2O3+Na2CO3=2NaFeO2+CO2 (7)
FeCr2O4+4NaFeO2+7/4O2=2Na2CrO4+5/2Fe2O3 (8)
MgO+Fe2O3=MgFe2O4 (9)
为弄清铬铁矿氧化焙烧过程的反应机理,对铬铁矿在1 323 K温度下不同焙烧时间得到的熟料进行了物相分析,不同焙烧时间熟料的XRD谱如图6所示。
由图6可以看出,在整个氧化焙烧过程中没有Na2Cr2O4生成,这一方面说明Na2CrO4生成过程并不一定经过反应式(6)生成Na2Cr2O4这一步骤,另一方面这也进一步证明氧气的扩散不是铬氧化反应的控制步骤(氧气不足时有Na2Cr2O4生成)。在氧化焙烧初期铬铁矿和Na2CO3迅速被消耗,焙烧反应前20 min内Na2CO3基本上反应完全,铬铁矿中铬的氧化率达到90%以上,主要生成物为Na2CrO4、MgFe2O4、MgO和NaFeO2;在氧化焙烧后期,氧化焙烧初期生成的NaFeO2逐步减少,熟料的XRD中,NaFeO2的特征峰逐渐减弱直至消失,铬完全氧化后熟料的主要物相组成为Na2CrO4、MgFe2O4和MgO等。
将熟料的物相变化规律和铬氧化反应动力学结果进行对比可以发现,熟料中Na2CO3反应完全的时间和氧化反应动力学特征函数发生转折的时间一致,此前Na2CO3逐渐被消耗生成Na2CrO4和NaFeO2,Na2CO3完全反应后,NaFeO2逐渐减少,铬氧化率缓慢升高。由此可推断铬的氧化反应机理为:氧化焙烧初期,铬铁矿中的铬尖晶石与碳酸钠和氧气通过反应(5)迅速生成Na2CrO4,Na2CO3在参与铬氧化反应的同时与铬铁矿中的部分铁化合物通过反应式(7)生成NaFeO2。由于铬铁矿氧化焙烧过程中Na2CrO4比NaFeO2更稳定[11],在氧化气氛下NaFeO2可通过反应式(8)与铬铁矿反应生成Na2CrO4,当焙烧过程中Na2CO3被消耗完时,氧化反应初期生成的NaFeO2将代替Na2CO3与未反应的铬铁矿反应生成Na2CrO4,使铬的氧化反应从反应式(5)转变为反应速度较慢的反应式(8)。
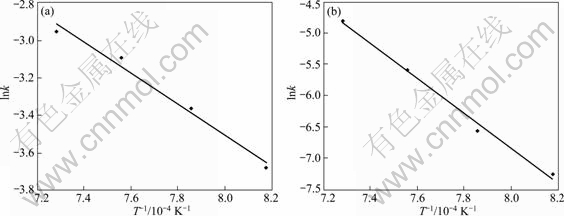
图5 铬氧化过程表观反应速率常数与温度之间的关系
Fig. 5 Relationship between apparent reaction rate constant and temperature: (a) Initial stage; (b) Later stage

图6 在1 323K下焙烧不同时间所得熟料的XRD谱
Fig. 6 XRD patterns of sinter obtained at 1 323K for different roasting times
为进一步验证铬铁矿氧化焙烧后期铬的氧化反应机理,采用非定温热分析方法对铬尖晶石与NaFeO2和氧气之间的氧化反应动力学进行了实验研究。在实验过程中为保证铬尖晶石与NaFeO2的反应环境与铬铁矿氧化焙烧后期的反应环境相同,实验所用的原料为NaFeO2和来自某厂的无钙焙烧铬渣。铬渣含铬9.2%(以Cr2O3计),其主要物相组成是铬铁(镁)尖晶石、MgO和MgFe2O4,反应环境与铬铁矿氧化焙烧后期基本相同。将铁酸钠与铬渣的混合物,在空气气氛下,以15 K/min的升温速度进行热重分析,根据热重曲线可以计算出各温度下铬的氧化率。
根据热分析动力学分析方法,利用Coats-Redfern温度积分法对热分析数据进行处理,取反应机理函数为F(x)=1-(1-x)1/3时,反应活化能可根据式(10)确 定[16]:
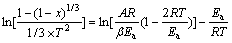 (10)
(10)
式中:x为铬氧化率;T为绝对温度,K;A为指前因子;R为摩尔气体常数,R=8.314 J/mol;Ea为表观反应活化能,J/mol; β为升温速度K/min;
在公式(5)中,对大部分化学反应而言,Ea/(RT)?1,即(1-2RT/Ea)≈1,因此公式右端第一项几乎为常数,则ln[(1-(1-x)1/3)/(1/3T2)]对1/T作图将得到一条直线,其斜率为-Ea/R,由此可得到反应的表观活化能。将在1 273~1 473 K温度范围内通过热分析计算得到的铬氧化率代入式(10)的左端,并对1/T作图,结果如图7所示。由图7求出的铬尖晶石与NaFeO2和氧气反应的表观反应活化能为237.3 kJ/mol,这与前述铬铁矿氧化焙烧动力学分析过程求得的铬氧化反应后期的

图7 ln[(1-(1-x)1/3)/(1/3T2)]与1/T关系图
Fig.7 Relationship between ln[(1-(1-x)1/3)/(1/3T2)] and reciprocal of temperature under constant heating rate
表观反应活化能基本相同。由此可进一步确定,在氧化焙烧后期,铬的氧化反应机理是铬铁矿与NaFeO2和氧气发生反应生成Na2CrO4。
由以上铬氧化动力学和反应机理的分析可知,在铬铁矿氧化焙烧初期,铬尖晶石直接与碳酸钠发生氧化反应,该阶段的表观反应活化能低,反应速度快,而在氧化焙烧后期,铬的氧化是通过铬尖晶石与铁酸钠发生氧化反应进行,该阶段的表观反应活化能高,反应速度慢且需要在较高温度下才能使铬氧化完全。因此,在研究强化铬铁矿氧化焙烧措施时,应对铬铁矿氧化焙烧后期铬的氧化反应给予足够重视。
3 结论
1) 铬氧化焙烧过程中,温度对铬氧化速率的影响较大,温度越高铬氧化速率越快。氧化焙烧初期铬氧化速率快,后期氧化速率慢。
2) 铬氧化焙烧分为初期和后期两个阶段,均受化学反应控制,铬氧化反应初期和后期的表观反应活化能分别为68.7和231.8 kJ/mol。
3) 铬氧化焙烧初期和后期的反应机理不同:氧化焙烧初期,铬尖晶石与Na2CO3和氧气直接反应生成Na2CrO4,Na2CO3在参与铬氧化反应的同时与铬铁矿中的铁化合物反应生成NaFeO2;氧化焙烧后期,铬尖晶石与NaFeO2和氧气发生反应生成Na2CrO4。
REFERENCES
[1] 丁 翼. 中国铬盐生产状况与展望[J]. 化工进展, 2004, 23(4): 345-348.
DING Yi. Present status and prospect of chromate production in China[J]. Chemical Industry and Engineering Progress, 2004, 23(4): 345-348.
[2] TATHAVADKAR V D, ANTONY M P, JHA A. The physical chemistry of thermal decomposition of south African chromite minerals[J]. Metallurgical and Materials Transactions B, 2005, 36B(1): 75-84.
[3] JOHN M, KUBICEK D L. Sodium chromate and sodium dichromate production capacities[J]. Encyclopedia of Chemical Process and Design, 1979, 8: 303-323.
[4] MOON D H, WAZNE M, DERMATAS D. Long-term treatment issues with chromite ore processing residue (COPR): Cr6+ reduction and heave[J]. Journal of Hazardous Materials, 2007, 143(3): 629-635.
[5] BEWLEY R J F. Calcium polysulfide remediation of hexavelant chromium contamination from chromite ore processing residue[J]. Science of the Total Environment, 2006, 364(1/3): 32-44.
[6] SUNGUR S, BABAOGLU S. Investigation of the effects of some parameters in the production of chromate from chromite ores in Iskenderun region[J]. Steel Research, 2006, 77(7): 492-494.
[7] XU Hong-bin, ZHANG Yi, LI Zuo-hu. Development of a new cleaner production process for producing chromic oxide from chromite ore[J]. Journal of Cleaner Production, 2006, 14(2): 211-219.
[8] ANTONY M P, JHA A, TATHAVADKAR V D. Alkali roasting of Indian chromite ores: thermodynamic and kinetic considerations[J]. Mineral Processing and Extractive Metallurgy, 2006, 115(2): 71-80.
[9] 纪 柱. 铬铁矿无钙焙烧的反应机理[J]. 无机盐工业, 1997, 18(l): 8-21.
JI Zhu. The reaction mechanism of chromite in non-lime roasting process[J]. Inorganic Chemicals Industry, 1997, 18(l): 8-21.
[10] RAMOS S S, CARBO A D, ADELANTADO J V, VICENTE J P. Analytical and mineralogical studies of ore and impurities from a chromite mineral using X-ray analysis, electrochemical and microscopy techniques[J]. Talanta, 2008, 74(5): 1592-1597.
[11] TATHAVADKAR V D, ANTONY M P, JHA A. The effect of Salt-Phase composition on the rate of soda-ash roasting of chromite ores[J]. Metallurgical and Materials Transactions B, 2003, 34(5): 555-563.
[12] BRUEN, CHARES P, WILLIANM W, SMALLEY, EDMUND W. Process for the production of sodium chromate from chromite ore: US3852059[P]. 1974.
[13] ARNDTA U, BATZA M, BELLINGHAUSENA R, BLOCKA H D, HELKERA H, LONHOFFA N, MORETTOA H H, NIEDER V H G, RINKESA H, SPRECKEL B, WEBERA R. Method for manufacturing alkaline chromates from chromium ore[J]. Minerals Engineering, 1996, 9(11): 1183.
[14] 李小斌, 齐天贵, 彭志宏, 刘桂华, 周秋生. 含铬物料强氧化焙烧技术: CN101456589[P]. 2009.
LI Xiao-bin, QI Tian-gui, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng. A technology for intensified oxidative roast of chromium-bearing materials: CN101456589[P]. 2009.
[15] 孙 康. 宏观反应动力学及其解析方法[M]. 北京: 冶金工业出版社, 1998.
SUN Kang. Macro-kinetics and Analytical Method[M]. Beijing: Metallurgical Industry Press, 1998.
[16] 胡荣祖, 史启桢. 热分析动力学[M]. 北京:科学出版社, 2001.
HU Rong-zu, SHI Qi-zhen. Thermal analysis kinetics[M]. Beijing: Science Press, 2001.
(编辑 何学锋)
基金项目:湖南省科技重大专项资助项目(2009FJ1009)
收稿日期:2008-12-29;修订日期:2010-04-01
通信作者:齐天贵,博士;电话:0731-88830453,Email:qtg_csu@163.com