J. Cent. South Univ. (2012) 19: 312-318
DOI: 10.1007/s11771-012-1006-5
In-situ growth of silver nanostructure on quartz glass substrates
YI Zao(易早)1, 2, ZHANG Jian-bo(张建波)1, NIU Gao(牛高)1, CHEN Yan(陈艳)1, 2,
LUO Jiang-shan(罗江山)1, WU Wei-dong(吴卫东)1, YI You-gen(易有根)2, TANG Yong-jian(唐永建)1, 3
1. Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China;
2. School of Physical Science and Technology, Central South University, Changsha 410083, China;
3. Institute of Atomic and Molecular Physics, Sichuan University, Chengdu 610065, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Silver nanostructure compact aggregates on the surface of quartz glass substrates were synthesized by small gold seeds with the assistance of poly vinypyrrolidone (PVP) and irradiation of fluorescent lamp. The formation mechanism of silver nanostructure was proposed. The results show that both the PVP and the light irradiation are the keys to in-situ growth of silver nanostructure on quartz glass substrates. The silver nanostructure of the substrates which finally grow up to 150 nm after 20 h irradiation exhibits irregular shape, and some of nanoparticles stack to form bilayer. A new broad band appears in the absorption spectra of the substrates due to the interparticle dipole-dipole coupling of surface plasmon resonance response of the triangular silver nanoplate particles, which red shifts 600-800 nm as the particles grow up. The substrates have an emission band centered at 400 nm on their fluorescence spectra, and the fluorescence intensity shrinks as the average size of the silver nanostructure increases. The strongest SERS signal of SERS-active substrate is fabricated after 16 h.
Key words: silver nanostructure; seed-mediated method; glass substrate; surface plasmon resonance; fluorescence; surface enhanced Raman scattering
1 Introduction
Construction of metallic nanoparticles has attracted significant attentions in the past decade because of their broad applications in catalysis, photonics, electronics and biosensors [1-4]. The properties of such assembled structures can be tailored by composition, geometry and spatial arrangement of the nanoparticles. Ag nanostructure has been extensively studied and becomes one of the best established systems particularly for investigation of size and shape effects on surface plasmon resonance (SPR) phenomenon on biosensing, such as the surface enhanced Raman scattering (SERS) [5]. Incorporation of Ag nanoparticles into a film is also an efficient way to construct antibacterial biomaterials [6]. Although a number of procedures have been developed to synthesize monodispersed silver nanoparticle or nanoprisms, a great challenge still remains to explore an effective, simple and economic method for construction of well organized nanoparticles on flat-surface substrates and devices for various applications.
Now, the growth of metallic nanoparticles on flat-surface substrates still requires costly equipment or complex approach, for example, the thermal evaporation, vacuum evaporation aggradation and electrochemical plating [7-10]. Without the help of costly equipment or complex approach, the nanoparticles deposited on liquid/ solid or gas/solid interfaces usually form island-like structures or particle films. Thus, there is still a need for much easier and more affordable techniques for the deposition of metal nanoparticles on planar surfaces. Recently, the photoreduction method has attracted many attentions. GEDVILAS et al [11] studied the thin metal films under intensive laser irradiation. In their work, gold, silver, copper, chromium and aluminum films with thickness of 100 nm were deposited on the glass substrate. Other examples include the deposition of silver nanoparticles on conventional glass substrates by JIA et al [12-13] and ZHAO et al [14].
In this work, the growth of silver nanoparticles on conventional quartz glass substrates, analogous to the wet-chemical method that was applied for the growth of silver nanoplates by JIA et al [13] on glass substrates, was reported. This amendatory approach enables the silver nanoparticles with compact aggregates to be adsorbed on the surface of glass substrates after irradiation of silanized quartz glass substrates attached with gold seeds smaller than 10 nm by a fluorescent lamp. All experiments were conducted at room temperature. The effect of surface morphology of silver nanoparticle thin films on the SERS activity of the substrate was studied by transmission electron microscopy, scanning electron microscopy, UV-Vis and fluorescence spectroscopy.
2 Experimental
Au seeds were prepared as follows: 0.6 mL of 0.1 mol/L NaBH4 solution was injected into 20 mL of 0.5 mmol/L aqueous mixture of Na3-citrate and HAuCl4 in ice bath under stirring. The as-prepared Au colloid was continually stirred for 3 h to degrade the excessive NaBH4.
Quartz glass substrates were cleaned by ultrasonication consecutively in ultra-pure water, ethanol, acetone, chloroform, acetone, ethanol, ultra-pure water for 5 min, respectively. Then, they were cleaned with “piranha solution” (30% (volume fraction) hydrogen peroxide/concentrated sulfuric acid) for at least 2 h. Finally, they were rinsed extensively with deionized water and dried in a stream of dry nitrogen. Glass substrates (1 cm × 1 cm) were prepared by silanized method reported by ASLAN et al [15]. APS-coated quartz substrates were immersed in the Au seed solution for 2 h and rinsed with deionized water, and then dried in a stream of nitrogen gas. In the reduction process, the growth solution contains 30 mL of 0.5 mmol/L AgNO3 and 0.5 mL of 2 mmol/L PVP aqueous mixture. The Au-seed-coated glass substrates were immersed in these aqueous solutions, then the mixtures were irradiated under a 27 W home fluorescent lamp for 20 h at ambient temperature without stirring.
The images of transmission electron microscope (TEM) were obtained with a JEM-2010 microscope at an accelerating voltage of 120.0 kV and the samples were prepared by placing a drop of aqueous dispersion products on carbon-coated copper grid. The scanning electron microscope (SEM) images were recorded using a Leica Cambridge S440 field emission scanning electron microscope at an accelerating voltage of 5.0 kV. The absorption spectra of these products were measured by a Perkin-Elmer Lambda 12 spectrophotometer. The fluorescence spectroscopy was obtained from a Hitachi F-4500 fluorescence spectrophotometer. The microscope attachment was based on a Leica DMLM system, and a 50× objective len was used to focus the laser beam onto a spot with approximately 1 μm in diameter. Radiation of 514.5 nm from an air cooled argon ion laser (Spectra- Physics Model 163-C4260) was used for excitation. All of the spectra reported were the results of a single 20 s accumulation.
3 Results and discussion
3.1 TEM and SEM images analysis
TEM image of the initial Au seeds is shown in Fig. 1(a). The Au seeds are spheroidal particles with average size of (4.2±0.6) nm. The Au seed solution is stored at 4 °C for one month. The Au seeds are electronegative because of adsorbed tri-sodium citrate, and the surface of silanized quartz glass substrate is antielectronegative because of adsorbed amido [15]. The Au seeds are easily adsorbed to the surface of quartz glass substrate by electrostatic adsorption. If the held mercapto and amido molecule matter are used instead of APS, the experiment method developed here can be extended to the fabrication of nanostructures of other metallic materials and can also be applied to others substrates. SEM image of the adsorbed Au seeds on quartz glass substrates is shown in Fig. 1(b). The Au seeds are homogeneously adsorbed to the surface of quartz glass substrate by electrostatic adsorption. These seeds offer advantaged condition for the in-situ growth of silver nanostructure.
Figure 2 shows the SEM images of the substrates irradiated for different time. The silver nanostructures are spheroidal particles with average size of 10 nm, arranging comparatively compact after 1 h irradiation. The average size of silver nanoparticles gradually becomes larger with the irradiation time. Figure 2(e) shows SEM images of substrate irradiated for 16 h. The silver nanostructure particles are spheroidal and irregular with average size of 100 nm, arranging comparatively compact. Some secondary homogenous nucleation particles lay on top of the first layer to form bilayer. After irradiated for 20 h, as shown in Fig. 2(f), more particles lay over the first layer, and the average size of the particles is 150 nm.

Fig. 1 TEM image of gold seeds and SEM image of glass substrate adsorbed with gold seeds: (a) TEM image; (b) SEM image

Fig. 2 SEM images of substrate irradiated for different time: (a) 1 h; (b) 4 h; (c) 8 h; (d)12 h; (e) 16 h; (f) 20 h
3.2 UV-Vis absorption spectra of substrates irradiated for different time
The growth of the products is monitored by UV-Vis spectroscopy. Figure 3 shows the SPR absorption spectra of products irradiated for different time. The SPR absorption bands of metal nanoparticles are produced by collective oscillation of the particles, and the conduction band electrons of surface particles are driven by photo-electric field. The peak absorption is affected by the morphology and size of the particles, the dielectric constant of the medium, the character of particle surface- coupled molecules, the degree of aggregation of particles and other factors [16]. As shown in Fig. 3, the absorption bands of the products are at 450 nm with meek shoulder bands. These absorption bands are attributed to the SPR absorption of irregular silver nanoparticles. They red shift gradually with the increase of irradiation time because the size of silver nanoparticles becomes larger. The products appear strong absorption bands at long wavelength, however, the absorption bands of products irradiated for 1 and 2 h are not obvious because these bands are close to the SPR peak of silver. The products irradiated for 20 h are immerged in water ultrasonic vibration for 90 s. The parts of the particles in water form light purple solution. The absorption bands appear at 450 nm only, and the strong absorption bands of products are not attributed to in-plane dipolar band nor the SPR absorption band of silver nanoparticles [17]. Preliminary studies showed that the strong absorption bands are attributed to the collective oscillation and the surface particles of conduction band electrons are driven by photoelectric field. The increased particles size and the reduced particles distance will lead to the red shift and strengthening of absorption band [18]. As shown in Fig. 3, the absorbance of the strong absorption bands is higher than the absorbance of the SPR absorption band, leading to the SPR absorption band becoming shoulder band. These indicate that the response of the dipolar action of near neighbour silver is stronger than the SPR absorption band of Au nanoparticles. The strong absorption bands red shift with the irradiated time. As the irradiated time increases, the strong absorption bands can be tuned from 450 nm to 812 nm. Because the size of the nanoparticles increases with the irradiated time increasing, the gap between the particles becomes narrow and particles arrange more closely, even some particles lay on the first layer, forming a localized high- density particle aggregates. These reasons accelerate the inter-particle dipole coupling growth, which can also be verified from the SEM images.
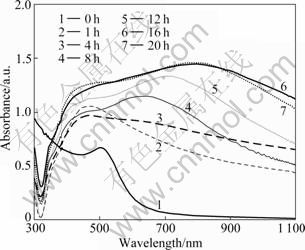
Fig. 3 Absorption spectra of substrates irradiated for different time
The absorbance of absorption bands of products irradiated for 16 h and 20 h is very close, which indicates that silver salt precursor is deoxidized in solution after 16 h. The SPR band of products irradiated for 20 h is stronger and red shifts compared with that of 16 h. The reason is that the solution cannot offer new atoms for the particles of substrate when silver salt precursor has been deoxidized completely. The absorption spectra of particles in the near-infrared light overlap with the fluorescent emission band of fluorescent lamp. The silver particles on the substrate turn laser energy into heat energy through surface plasmon resonance absorption. The local temperature of substrate rises high, making the particles contact with each other and trigger Ostwald reaction [19]. The atoms of small particles transfer to large particles through solution as medium, resulting in the proportion of large particles increasing and the average size of the particles filling out. So the shoulder of 450 nm peak moves higher and the in-plane dipolar SPR absorption band blue shifts.
3.3 Fluorescence emission spectra of substrates for different irradiation time
MOHAMED et al [20] found that two modes many contribute to metal nanoparticles irradiance: A1 mode makes the exterior electro-plasma of particles resonate, and the resonance emit fluorescence; B1 mode makes the d or sp bands of electron of particles excitate transition. The cavities are left after transition. These cavities can hold electrons to form exciton easily because of the smaller size of nanoparticles and the shorter movement distance of electron, so the concentration of exciton is much larger. The exciton bands are formed at the bandgap near the bottom of conduction by quantum confinement effect, and these exciton bands can induce the compound of electron and cavity to emit fluorescence. In the present work, the SPR absorption bands of substrates locate at 430-450 nm and near-infrared, and both fluorescence and emission wavelengths are apart from the area. So, the producing mechanism of fluorescence is B1 mode. Figure 4 shows the fluorescence spectra of the substrates for different irradiated time. There are mainly three peaks on the fluorescence spectra. The first peak locates between 300 nm and 380 nm (photoluminescence 1, short for PL1), the second peak locates between 480 nm and 530 nm (PL2), and the third peak locates between 600 nm and 650 nm (PL3). The peaks at 430 nm and 645 nm are the excitations of 1/2 and 1/3 fraction frequency, respectively. The excitation at 215 nm makes the d or sp bands of electron transition of particles produce cavity. The electron on Fermi energy level recombined with the radiation of cavity emits fluorescence. As shown in Fig. 4, there exist the recombination of three formal bands, corresponding to three emission peaks on spectra, respectively. The fluorescence of noble metal nanoparticles correlates with the size of nanoparticles. When the size of nanoparticles is small, incidence ray together with the interparticle dipole-dipole coupling of surface plasmon resonance response of particles eradiates fluorescence. The large size of the particles will make the efficiency of coupling decrease when the intensity of fluorescence is decreased, because the surface configuration of particles changes [21]. As shown in Fig. 4, the fluorescence intensity shrinks as the average size of the silver nanoparticles increases.
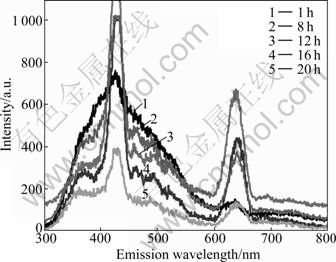
Fig. 4 Fluorescence emission spectra of substrates for different irradiated time
3.4 Raman spectra of R6G on substrates for different irradiated time
Rhodamine 6G is chosen as an analyte because it has been well characterized by SERS and by resonance Raman spectroscopy. The SERS spectra of single R6G molecules have been obtained [22] and most of the prominent Raman bands have been assigned. The 514.5 nm line from an argon ion laser is used in the present work. This wavelength corresponds to a resonant excitation of R6G, so the surface-enhanced resonance Raman spectrum (SERRS) is acquired. The SERRS is accepted that the increase in signal is due to an increase in the apparent cross-section of the molecules. So, when estimating the SERS enhancement factor, the non-SERS cross-section should be considered first. R6G is a dye that adsorbs strongly with a maximum at 528 nm and is close to a resonance Raman scattering at 514.5 nm laser excitation [22]. The experimental resonance Raman scattering spectrum is difficult to obtain because of the overwhelming fluorescent background at 514.5 nm. Figure 5 shows the SERS spectra that the R6G molecules are adsorbed onto the substrates for different irradiated time. The SERS spectra show the resonance Raman scattering of the R6G deposited film that is prepared by dropping 20 μL R6G methanol solution (10-6 mol/L) onto the substrates. As shown in Fig. 5, when the R6G methanol solution is dropped onto the Au-seed-coated glass substrates, as expected, the signal is very weak. However, when the R6G methanol solution is dropped onto the products for different irradiated time, the SERS signals become strong. That is because the intrinsic activity of Ag nanoparticles is much higher than Au nanoparticles. Several strong bands at 1 650, 1 598, 1 574, 1 507, 1 361, 1 310, 1 187, 774 and 611 cm-1 are observed on the substrate. The bands at 1 650, 1 574, 1 507 and 1 361 cm-1 are assigned to aromatic C—C stretching; the bands at 1 598 and 1 130 cm-1 are assigned to C=C stretching; the bands at 1 187 and 611 cm-1 are assigned to aromatic C—H bending and aromatic bending, respectively [23]. As the irradiated time increases, the SERS signal of SERS-active substrate becomes stronger. The hot sites increase because the size of the nanoparticles increases with the irradiated time increasing, and the dipole-dipole coupling of inter- particle pricks up. The strongest SERS signal of SERS-active substrate is fabricated after 16 h. When the irradiated time increases to 20 h, as shown in Fig. 5, the SERS signal of SERS-active substrate decrease. The hot sites decrease, because the increasing size of the nanoparticles makes the silver nanoparticles decrease in unit laser irradiated area.
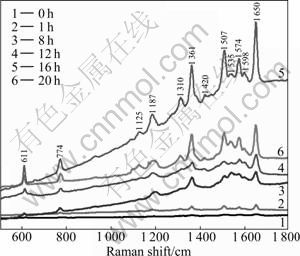
Fig. 5 Raman spectra of R6G on substrates for different irradiated time
3.5 Analysis of mechanism
The construction process is schematically shown in Fig. 6. It is found that both the PVP and the light irradiation are the keys to in-situ growth of silver nanostructure on quartz glass substrates.
In the cases without PVP but with light irradiation, the UV-Vis absorption spectra of products after 20 h irradiation superpose with the products without light irradiation. This indicates that PVP in water is able to reduce Ag+ to Ag. WASHIO et al [24] proved that the carboxyl is produced at the aggregation of the bottom of PVP molecule chain. The carboxyl is moderation reducing agent at the reaction of noble metal saline. KAN et al [25] found that the lone pair of electrons from the nitrogen and oxygen atoms in the polar groups of the PVP repeated unit was donated into sp hybrid orbits of Ag+ ions to construct complex compounds, so as to effectively decrease chemical potential and further enable the PVP-bound Ag+ ions to be reduced more easily. In the cases without light irradiation but with PVP added, the deep pink products appear, but does not become blue after 2 d. The absorbance of absorption spectra of products becomes stronger and red shifts slightly. This indicates that the silver atoms are deposited on the surface of Au seeds, and the deoxidize velocity of PVP vs Ag+ is very slow [26], so the growth of nanoparticles is very slow. These imply that light could enhance the reduction power of those systems. Thus, it is necessary to bring in light irradiation so as to produce Ag particles onto quartz glass substrates in high yield.
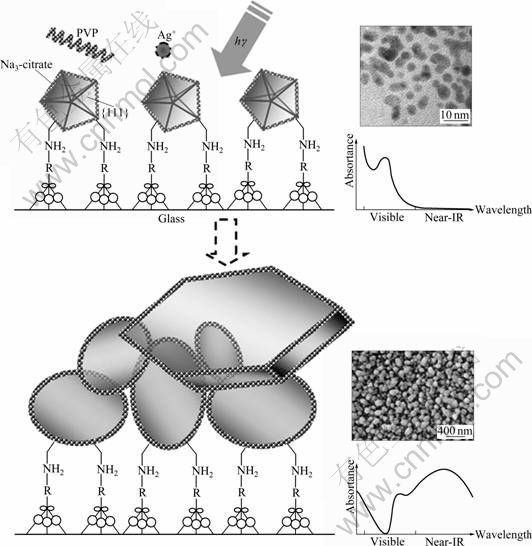
Fig. 6 Schematic illustration of mechanism
4 Conclusions
1) Silver nanostructures on the surface of glass substrate have been synthesized by small gold seeds with the assistance of poly vinypyrrolidone and irradiation of a fluorescent lamp. The formation mechanism of silver nanostructure is proposed. Both the PVP and the light irradiation are the keys to in-situ growth of silver nanostructure on quartz glass substrates.
2) The silver nanostructure of the substrates which finally grows up to 150 nm after 20 h irradiation takes on irregular shape, and some of nanoparticles stack to form bilayer. A new broad band appears on the absorption spectra of the substrates due to the interparticle dipole-dipole coupling of surface plasmon resonance response of the triangular silver nanoplate particles, which red shifts 600-800 nm as the particles grow up. The substrates have an emission band centered at 400 nm on their fluorescence spectra, and the fluorescence intensity shrinks as the average size of the silver nanostructure increases. The strongest SERS signal of SERS-active substrate is fabricated after 16 h irradiation. The synthetic strategy may provide a convenient way to synthesize other noble metals with planar structures. Furthermore, in-situ growth of silver nanostructure makes localized high-density particle aggregates form on quartz substrates.
References
[1] BHUPENDRA C, ANJANA K V, NIDHI A, MEHTA R V, UPADHYAY R V. Highly bacterial resistant silver nanoparticles: Synthesis and antibacterial activities [J]. J Nanopart Res, 2010, 12(3): 1677-1685.
[2] NELAYAH J, KOCIAK M, GEUQUET N, COLLIEX C. Two- dimensional quasistatic stationary short range surface plasmons in flat nanoprisms [J]. Nano Lett, 2010, 10(1): 902-907.
[3] SANTANA A C, ROCHA T C R, SANTOS P S, ZANCHET D, TEMPERINI M L A. Size-dependent SERS enhancement of colloidal silver nanoplates: The case of 2-amino-5-nitropyridine [J]. J Raman Spectrosc, 2009, 40(1): 183-190.
[4] CHETNA D, MAUMITA D, GAJJALA S, AVANISH K S, MANOJ K D, CHEOL G K, MONIKA D, BANSI D M. Preparation, characterization and application of polyaniline nanospheres to biosensing [J]. Nanoscale, 2010, 2(1): 747-754.
[5] ZHOU J, XU S P, XU W Q, ZHAO B. In situ nucleation and growth of silver nanoparticles inmembranematerials: A controllable roughened SERS substrate with high reproducibility [J]. J Raman Spectrosc, 2009, 40(1): 31-37.
[6] CUI X Q, LI C M, BAO H F, ZHENG X T, LU Z S. In situ fabrication of silver nanoarrays in hyaluronan/PDDA layer-by-layer assembled structure [J]. Journal of Colloid and Interface Science, 2008, 327(1): 459-465.
[7] HUANG Qian, WANG Jing, CAO Li-ran, SUN Jian, ZHANG Xiao-dan, GENG Wei-dong, XIONG Shao-zhen, ZHAO Ying. Research of surface enhanced Raman scattering caused by surface plasmon of Ag nanostructures [J]. Acta Physica Sinica, 2009, 58(3): 1980-1985. (in Chinese)
[8] NISHIKAWA T, NAKANO H, OGURI K, UESUGI N, NAKAO M, NISHIO M, MASUDA H. Nanocylinder-array structure greatly increases the soft X-ray intensity generated from femtosecond-laser- produced plasma [J]. Appl Phys B, 2001, 73(2): 185-188.
[9] LIU G Q, CAI W P, LIANG C H. Trapeziform Ag nanosheet arrays induced by electrochemical deposition on Au-coated substrate [J]. Cryst Growth Des, 2008, 8(8): 2748-2752.
[10] TIAN Zong-jun, WANG Gui-feng, HUANG Yin-hui, LIU Zhi-dong, CHEN Jin-song. Fractal growth of Ni dendrite in electrodeposition [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(1): 167-173. (in Chinese)
[11] GEDVILAS M, VOISIAT B, RACIUKAITIS G, REGELSKIS K. Self-organization of thin metal films by irradiation with nanosecond laser pulses [J]. Applied Surface Science, 2009, 255(6): 9826-9829.
[12] JIA H Y, ZENG J B, AN J, SONG W, XU W Q, ZHAO B. Preparation of silver nanoparticles by photo-reduction for surface-enhanced Raman scattering [J]. Thin Solid Films, 2006, 496(2): 281-287.
[13] JIA H Y, ZENG J B, AN J, SONG W, XU W Q, ZHAO B. Preparation of triangular and hexagonal silver nanoplates on the surface of quartz substrate [J]. Thin Solid Films, 2008, 516(7): 5004-5009.
[14] ZHAO J W, TIAN R H, ZHI J F. Deposition of silver nanoleaf film onto chemical vapor deposited diamond substrate and its application in surface-enhanced Raman scattering [J]. Thin Solid Films, 2008, 516(6): 4047-4052.
[15] ASLAN K, JOSEPH R, CHRIS D G. Rapid deposition of triangular silver nanoplates on planar surfaces: Application to metal-enhanced fluorescence [J]. J Phys Chem B, 2005, 109(13): 6247-6251.
[16] YI Zao, TANG Yong-jian, YI You-gen, LI Kai, LUO Jiang-shan, LI Xi-bo, ZHANG Jian-bo, YE Xin. Preparation of hollow silver microspheres and their characterization [J]. High Power Laser and Particle Beams, 2009, 21(9): 1354-1359. (in Chinese)
[17] KELLY K L, CORONADO E, ZHAO L L, GEORGE C S. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment [J]. J Phys Chem B, 2003, 107(3): 668- 677.
[18] XU X, STEVENS M, CORTIE M B. In situ precipitation of gold nanoparticles onto glass for potential architectural applications [J]. Chem Mater, 2004, 16(3): 2259-2266.
[19] SUN Y, GATES B, MAYERS B, XIA Y N. Crystalline silver nanowires by soft solution processing [J]. Nano Lett, 2002, 2(2): 165-168.
[20] MOHAMED M B, VOLKOV V, LINK S, MOSTSFA A E. The ‘lightning’ gold nanorods: Fluorescence enhancement of over a million compared to the gold metal [J]. Chem Phys Lett, 2000, 317(6): 517-523.
[21] WILCOXON J P, MARTIN J E, PARSAPOUR F, WIEDENMAN B, KELLEY D F. Photoluminescence from nanosize gold clusters [J]. J Chem Phys, 1998, 108(17): 9137-9143.
[22] GROCHALA W, KUDELSKI A, BUKOWSKA J. Anion-induced charge-transfer enhancement in SEES and SERRS spectra of rhodamine 6G on a silver electrode: How important is it? [J]. J Raman Spectrosc, 1998, 29(8): 681-685.
[23] WEI G, ZHOU H L, LIU Z G, LI Z. A simple method for the preparation of ultrahigh sensitivity surface enhanced Raman scattering (SERS) active substrate [J]. Applied Surface Science, 2005, 240(1): 260-267.
[24] WASHIO I, XIONG Y, YIN Y, XIA Y. Reduction by the end groups of poly(vinyl pyrrolidone): A new and versatile route to the kinetically controlled synthesis of Ag triangular nanoplates [J]. Adv Mater, 2006, 18(6): 1745-1749.
[25] KAN C, CAI W, LI C, ZHANG L D. Optical studies of polyvinylpyrrolidone reduction effect on free and complex metal ions [J]. J Mater Res, 2005, 20(2): 320-324.
[26] JIANG P, LI S Y, XIE S S, GAO Y, SONG L. Machinable long PVP-stabilized silver nanowires [J]. Chem Eur J, 2004, 10(5): 4817- 4821.
(Edited by HE Yun-bin)
Foundation item: Projects(10804101, 60908023) supported by the National Natural Science Foundation of China; Project(2007CB815102) supported by the National Basic Research Program of China; Project(2007B08007) supported by the Science and Technology Development Foundation of Chinese Academy of Engineering Physics
Received date: 2010-11-26; Accepted date: 2011-03-24
Corresponding author: YI You-gen, Professor, Researcher; Tel: +86-816-2484233; E-mail: myyz1984@yahoo.cn