含铜砷的铜电解黑泥氧化酸浸-硫化沉淀分离和回收铜
来源期刊:中国有色金属学报(英文版)2021年第4期
论文作者:史美清 闵小波 沈忱 柴立元 柯勇 颜旭 梁彦杰
文章页码:1103 - 1112
关键词:铜电解黑泥;氧化酸浸;选择性硫化沉淀;浸出动力学;铜回收
Key words:copper electrorefining black slime; oxidation acid leaching; selective sulfide precipitation; leaching kinetics; copper recovery
摘 要:开发从含铜砷的铜电解黑泥中分离和回收铜的湿法冶金新工艺。该工艺包括黑泥氧化酸浸和浸出液中选择性硫化沉铜两个步骤。研究各种工艺参数对铜和砷的浸出和沉淀的影响。在第一阶段中,最佳工艺条件为:初始H2SO4浓度为1.0 mol/L,液固比为10 mL/g,80 °C下连续浸出4 h。此条件下铜浸出率可达95.2%,砷浸出率为97.6%。同时,通过Avrami模型成功模拟氧化酸浸过程铜和砷的浸出动力学,发现铜和砷浸出反应的表观活化能分别为33.6和35.1 kJ/mol,表明该浸出过程主要受化学反应和扩散混合控制。在选择性硫化沉淀过程中,最佳工艺条件为:硫与铜摩尔比2.4:1、时间1.5 h、温度25 °C。此条件下99.4%的铜以CuS形式回收,而砷的沉淀率仅0.1%。
Abstract: A new hydrometallurgical route for separation and recovery of Cu from Cu-As-bearing copper electro- refining black slime was developed. The proposed process comprised oxidation acid leaching of Cu-As-bearing slime and selective sulfide precipitation of Cu from the leachate. The effects of various process parameters on the leaching and precipitation of Cu and As were investigated. At the first stage, Cu extraction of 95.2% and As extraction of 97.6% were obtained at 80 °C after 4 h with initial H2SO4 concentration of 1.0 mol/L and liquid-to-solid ratio of 10 mL/g. In addition, the leaching kinetics of Cu and As was successfully reproduced by the Avrami model, and the apparent activation energies were found to be 33.6 and 35.1 kJ/mol for the Cu and As leaching reaction, respectively, suggesting a combination of chemical reaction and diffusion control. During the selective sulfide precipitation, about 99.4% Cu was recovered as CuS, while only 0.1% As was precipitated under the optimal conditions using sulfide-to-copper ratio of 2.4:1, time of 1.5 h and temperature of 25 °C.
Trans. Nonferrous Met. Soc. China 31(2021) 1103-1112
Mei-qing SHI1, Xiao-bo MIN1,2, Chen SHEN1, Li-yuan CHAI1,2, Yong KE1,2, Xu YAN1,2, Yan-jie LIANG1,2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China
Received 8 April 2020; accepted 3 September 2020
Abstract: A new hydrometallurgical route for separation and recovery of Cu from Cu-As-bearing copper electro- refining black slime was developed. The proposed process comprised oxidation acid leaching of Cu-As-bearing slime and selective sulfide precipitation of Cu from the leachate. The effects of various process parameters on the leaching and precipitation of Cu and As were investigated. At the first stage, Cu extraction of 95.2% and As extraction of 97.6% were obtained at 80 °C after 4 h with initial H2SO4 concentration of 1.0 mol/L and liquid-to-solid ratio of 10 mL/g. In addition, the leaching kinetics of Cu and As was successfully reproduced by the Avrami model, and the apparent activation energies were found to be 33.6 and 35.1 kJ/mol for the Cu and As leaching reaction, respectively, suggesting a combination of chemical reaction and diffusion control. During the selective sulfide precipitation, about 99.4% Cu was recovered as CuS, while only 0.1% As was precipitated under the optimal conditions using sulfide-to-copper ratio of 2.4:1, time of 1.5 h and temperature of 25 °C.
Key words: copper electrorefining black slime; oxidation acid leaching; selective sulfide precipitation; leaching kinetics; copper recovery
1 Introduction
Almost all copper from primary or secondary resources is deposited electrolytically at its final stage of production [1,2]. The electrolysis is performed to refine the impure copper anodes to produce pure copper cathodes. Although smelting, converting and refining greatly reduce the content of impurities, a small quantity of impurities still remain in the copper anodes. During the electrolytic refining of copper, the impurity elements such as As, Sb and Bi gradually dissolve and concentrate in the electrolyte [3-5]. When the concentrations of impurity elements reach a concentration detrimental to the electrolytic production of high-purity copper, continuous withdrawal and treatment of the refinery electrolyte is required to maintain the requisite electrolyte purity. During the decopperization of electrolyte by electrowinning, the arsenic deposits as a black slime along with copper. Such a Cu-As- bearing copper electrorefining black slime (Cu-As- bearing slime, for short) is characterized by high contents of copper and arsenic in the form of Cu-As compounds, such as Cu3As and Cu(HAsO4) [6,7]. Because of the toxic elemental arsenic and valuable elemental copper, the cleaning treatment of Cu-As-bearing slime is becoming important for copper smelters from the perspective of both resource utilization and environment protection.
Traditionally, the Cu-As-bearing slime is returned to the copper smelter to recovery copper because of its high copper content. However, this will lead to increased arsenic circulation in the smelting processes. With increasing stringent environmental regulation, this traditional treatment method is gradually being eliminated, and storage seems to be the only approach for managing Cu-As-bearing slime. On the other hand, the impurity content, especially arsenic grade, in copper ores has tended to increase year by year, and therefore the arsenic grade in copper concentrates obtained from copper ores is becoming increasingly higher. Thus, an increasing amount of Cu-As- bearing slime will be produced. As a result, the Cu-As-bearing slime has become a major problem in smelters in which the concentrates that contain arsenic are being processed.
Generally, materials containing arsenic can be treated by either pyrometallurgical or hydrometallurgical processes [8-11]. In the pyrometallurgical processes, arsenic is readily volatilized as As2O3 and collected in the flue dust [12,13]. Hydrometallurgical processes are based on acid leaching [14], alkaline leaching [15-17], pressure leaching [18], mechanical activation leaching [19], and so on. However, the existing methods for treating Cu-As-bearing slime are less than satisfactory, which presents many drawbacks, e.g., long treatment process, low efficiency, environmental pollution and high cost. An ideal process for the treatment of Cu-As-bearing slime should be a hydrometallurgical process, operating at low temperature and atmospheric pressure, with few unit operations and low energy costs, by which arsenic removal and copper recovery can be achieved simultaneously.
This work presents a hydrometallurgical route that consists of oxidation acid leaching and selective sulfide precipitation for processing Cu-As-bearing slime. The effect of various process parameters on acid leaching of Cu and As from Cu-As-bearing slime and subsequent separation and recovery of Cu from the leachate were studied. In addition, the leaching kinetics of acid leaching was also studied.
2 Experimental
2.1 Materials
The Cu-As-bearing slime used in this study was supplied by the Tongling Nonferrous Metals Group Co., Ltd., Anhui of China. The as-received material was crushed using a laboratory vibration mills to pass through a 75 μm sieve. This was followed by splitting of the crushed product to get representative samples for chemical analyses and mineralogical study. Chemical analysis of the Cu-As-bearing slime was performed by ICP-AES (IRIS Intrepid II XSP) after digestion, and the result is listed in Table 1. The contents of Cu and As in the Cu-As-bearing slime were 44.2% and 15.4%, respectively. As shown in Fig. 1, the main mineral components of the Cu-As-bearing slime are geminite (Cu(AsO3OH)(H2O)), copper sulfate hydrate (Cu(SO4)(H2O)), nantokite (CuCl) and domeykite (Cu3As). The presence of CuCl is due to the use of HCl as an additive to the electrolyte.
Table 1 Main elemental composition of Cu-As-bearing slime (wt.%)


Fig. 1 XRD patterns of Cu-As-bearing slime
2.2 Experimental procedure
The experiment procedure is shown in Fig. 2, which mainly consists of two unit operations, that is, oxidation acid leaching and selective sulfide precipitation. The operations for these two processes are described below.
2.2.1 Oxidation acid leaching

Fig. 2 Experiment process for separation and recovery of Cu

Fig. 3 Reactor of oxidation acid leaching
All leaching experiments were performed in a 500 mL 3-neck and round-bottom flask, as shown in Fig. 3. Magnetic stirring was used for mixing with a fixed speed of 400 r/min. The temperature was controlled by a thermostatic water bath. In each experiment, 20 g of the Cu-As-bearing slime was mixed with a known quantity of sulfuric acid solution in the flask. Reaction conditions were manipulated individually to identify the optimum leaching conditions, i.e., concentration of sulfuric acid (0.5-3.0 mol/L), reaction temperature (50-90 °C), liquid-to-solid ratio (L/S ratio, 6-14 mL/g), and reaction time (1-5 h). The sulfuric acid solution was first heated to the set reaction temperature. Then, the Cu-As-bearing slime was added to the reactor. Finally, air with a flow of 0.8 m3/h was fed to the solution and mixing was started. After leaching, the product solution was filtered and the solids washed with distilled water, yielding a leachate and a black leach residue.
2.2.2 Selective sulfide precipitation of copper
Selective sulfide precipitation of copper was conducted by introducing H2S into the diluted leachate, the feasibility of which was confirmed in our previous study by theoretical calculation as well as experiments using solutions with copper concentration of 600 mg/L and arsenic concentration of 2000 mg/L [20]. The entire experimental process was carried out in the apparatus that was divided into two parts, as shown in Fig. 4. The left part was used to generate H2S by the reaction between FeS and HCl, and sulfide precipitation occurred in the right part. A small hole was drilled in the partition to maintain the pressure balance on both sides. H2S was passed into the gas inlet of the jet device, and the leachate was pumped into the liquid inlet of the jet device, reacting and continuously circulating. After reacting for a certain period, the liquid sample was taken out and 1-2 mL of polyacrylamide solution (10 mg/L) was added to facilitate the separation of precipitate from solution. The effects of sulfide-to-copper ratio, reaction time and temperature on precipitation and separation of Cu from leachate were investigated. A stirring speed of 400 r/min was applied with a magnetic stirrer in all the experiments.

Fig. 4 Experimental apparatus of selective sulfide precipitation
2.3 Characterization and chemical analyses
The Cu and As concentrations in the solution were determined by ICP-AES. The leaching rates of Cu and As were calculated as previously described in Ref. [21]. The precipitation rates (R) of Cu and As were calculated according to Eq. (1):
 (1)
(1)
where C1 was Cu (or As) concentration in leachate obtained from the process of oxidation acid leaching, C2 was Cu (or As) concentration in the solution after sulfide precipitation.
The crystallographic composition of the precipitates were identified by X-ray diffraction (XRD, D/max2550 VB+ 18 kW), and the elemental compositions were analyzed by SEM-EDS (Nova NanoSEM 230).
3 Results and discussion
3.1 Oxidation acid leaching of Cu-As-bearing slime
3.1.1 Effect of initial H2SO4 concentration
The effect of initial H2SO4 concentration on the leaching of Cu-As-bearing slime was investigated at 80 °C for 4 h with L/S ratio of 10 mL/g. As shown in Fig. 5, the leaching rtes of Cu and As exhibited the same variation tendency with the variation of initial H2SO4 concentration. These increased remarkably as H2SO4 concentration increased from 0.5 to 1.0 mol/L, with a change from 60.9% to 95.0% for Cu and from 68.3% to 97.5% for As. In contrast, with enhancing acid concentration between 1.0 and 3.0 mol/L, the leaching rates were virtually constant. These remained stable at approximately 98% for As and 95% for Cu. Thus, the optimal initial H2SO4 concentration was determined to be 1.0 mol/L, and all the subsequent experiments were performed under this condition.
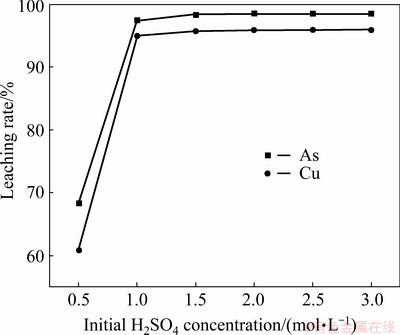
Fig. 5 Effect of initial H2SO4 concentration on leaching rates of Cu and As
3.1.2 Effect of temperature
The effect of temperature on the leaching of Cu-As-bearing slime was studied at the initial H2SO4 concentration of 1.0 mol/L, L/S ratio of 10 mL/g, and leaching time of 4 h. It can be seen from Fig. 6 that temperature was observed to have a significant effect on the leaching rates of Cu and As. The leaching rates of Cu and As increased from 68.8% to 95.5% and from 76.4% to 98.0%, respectively, when the temperature was elevated from 50 to 80 °C. However, there were no significant changes in the leaching efficiencies of Cu and As at an elevated temperature of 90 °C. Therefore, the optimal temperature was chosen to be 80 °C.

Fig. 6 Effect of temperature on leaching rates of Cu and As
3.1.3 Effect of liquid-to-solid ratio
Figure 7 presents the effect of the L/S ratio on the leaching of Cu-As-bearing slime at the initial H2SO4 concentration of 1.0 mol/L, temperature of 80 °C, and leaching time of 4 h. The leaching rates of Cu and As elevated rapidly as L/S ratio increased from 6 to 10 mL/g. When the L/S reached 10 mL/g, the leaching rates of Cu and As were 95.3% and 98.0%, respectively. However, further increase in L/S ratio was found to have no remarkable effect on the leaching rates of Cu and As. Hence, the optimal L/S was selected as 10 mL/g.
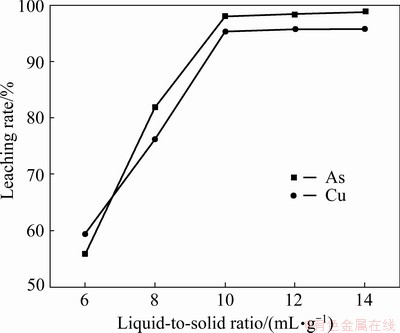
Fig. 7 Effect of liquid-to-solid ratio on leaching rates of Cu and As
3.1.4 Effect of leaching time
The effects of leaching time on the leaching rates of Cu and As at initial H2SO4 concentration of 1.0 mol/L, temperature of 80 °C and L/S of 10 mL/g are presented in Fig. 8. It can be seen that the leaching was efficient and fast with 64.4% of Cu and 75.4% of As extracted within 1 h. Thereafter, with prolonged leaching time, the leaching rates of Cu and As reached 95.6% and 98.1%, respectively, after 4 h. Further increase in the leaching time resulted in a gradual increase in the levels of Cu and As leaching rates, indicating that the leaching reaction gradually reaches equilibrium. Thus, 4 h was regarded as the optimal leaching time.
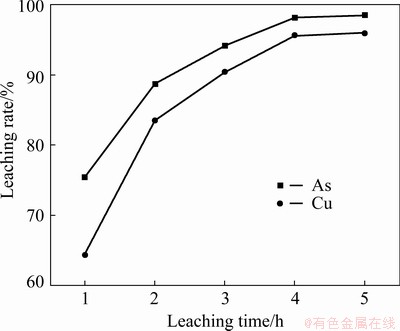
Fig. 8 Effect of leaching time on leaching rates of Cu and As
3.1.5 Optimum conditions
Based on the above results, the most suitable leaching conditions were determined to be an initial H2SO4 concentration of 1.0 mol/L, with L/S of 10 mL/g at 80 °C for 4 h. Under these conditions, confirmation experiments were conducted five times. The results and corresponding average values are shown in Fig. 9. It can be seen that the results of parallel experiments were similar with small standard deviation. Therefore, it was appropriate to choose these optimum conditions. Under the optimum conditions, the average leaching rates of Cu and As were 95.2% and 97.6%, respectively, and the average concentrations of Cu and As in the leachate were (43.8±0.2) and (15.1±0.1) g/L, respectively.
3.2 Kinetics of oxidation acid leaching
In order to undertake kinetic analysis of the oxidation acid leaching of Cu-As-bearing slime, the leaching rates of Cu and As as a function of time were examined at 50, 60, 70, 80 and 90 °C, respectively. The results are shown in Fig. 10.
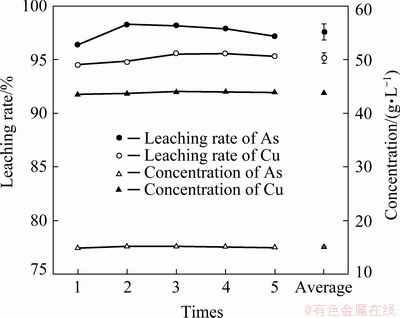
Fig. 9 Results of repeated experiments under optimal conditions
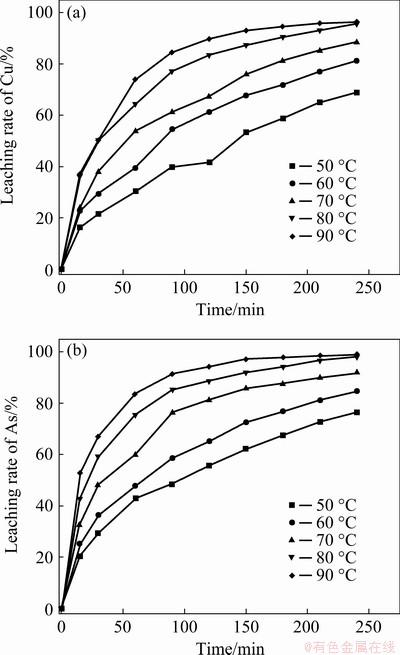
Fig. 10 Effect of temperature on leaching rates of Cu (a) and As (b)
The leaching reaction of Cu and As is a solid-liquid heterogeneous reaction taking place at the liquid-solid interface. The unreacted shrinking core model is most widely used to describe the solid-fluid heterogeneous reaction and determine the rate-controlling step [22,23]. However, a satisfactory fit was not obtained in this work when applying various shrinking core models to the experimental data. The regression coefficients (R2) for the chemical reaction control model and the diffusion control model are shown in Table 2.
Table 2 Regression coefficients (R2) for chemical reaction control and diffusion control shrinking core models at different temperatures

It can be seen that the regression analysis showed poor linearity. When fitted with chemical reaction control model, the linearity was good at low temperatures and became poor at high temperatures, and the fitting results with the diffusion control model was opposite. Therefore, the oxidation acid leaching kinetics of Cu-As- bearing slime could not be analyzed by single chemical reaction control or diffusion control model. It seemed to be controlled by a mixture of chemical reaction and diffusion. Considering the leaching rates of Cu and As as a function of time shown in Fig. 8, we inferred that the kinetics of such a liquid-solid reaction was in accordance with the Avrami model [24,25]. The kinetic equation of Avrami model is
-ln(1-X)=ktn (2)
where X is the leaching rate, k is the reaction rate constant and t is the reaction time. The parameter n is a function of properties and geometry of the solid particles and independent of process conditions. The value of n has been classified to three levels: for n<1, the initial rate is infinite but decreases with increasing time; for n=1, the initial rate is finite and the leaching process is controlled by chemical reaction; for n>1, the initial rate is close to zero [26]. Further, for 0.5≤n<1, the leaching process is a mixed type of chemical reaction and diffusion control; for n<0.5, it is controlled by diffusion process.
Based on the results of Fig. 10, the variations of ln[-ln(1-X)] with ln t were plotted for the reaction temperatures in Fig. 11. The good straight lines in Fig. 11 indicated that the leaching of Cu and As can be described by the Avrami model. The values of the regression coefficients and ln k calculated at various temperatures are given in Table 3. The average values of n for Cu and As leaching were calculated to be 0.7148 and 0.6641, respectively.
The apparent activation energies of the reaction were determined from the Arrhenius plot (Fig. 12) to be 33.6 kJ/mol for the Cu leaching reaction and 35.1 kJ/mol for As leaching reaction, which confirmed that the kinetics of oxidation acid leaching for Cu-As-bearing slime was controlled by both chemical reaction and diffusion. The similar apparent activation energies for Cu and As indicated their similar leaching reaction processes, which can be contributed to the primary phases of Cu-As compounds, such as geminite (Cu(AsO3OH)(H2O)) and domeykite (Cu3As) in Cu-As-bearing slime.
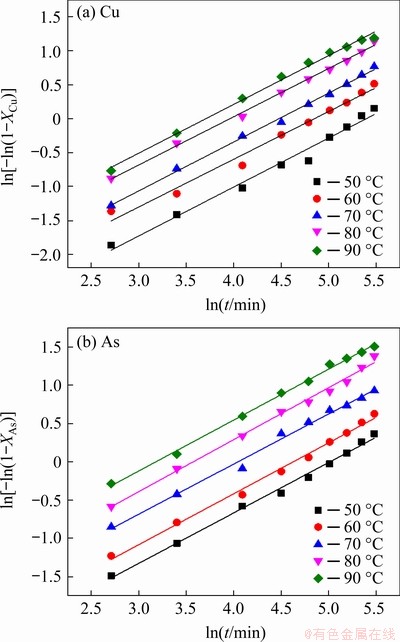
Fig. 11 Variation of ln[-ln(1-X)] vs ln t at different reaction temperatures
Table 3 Correlation coefficient of Avrami model at different temperatures

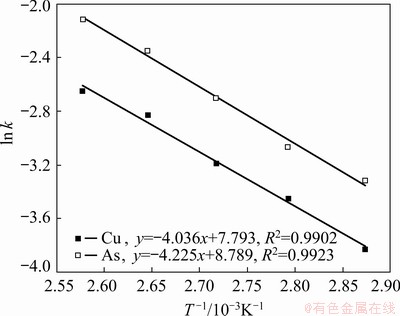
Fig. 12 Arrhenius plot of Avrami model reaction rates for As and Cu extractions against reciprocal temperature
3.3 Selective sulfide precipitation of Cu in leachate
3.3.1 Effect of sulfide-to-copper molar ratio
The effects of sulfide-to-copper molar ratio on the precipitation rates of Cu and As from the leaching solution were investigated at 25 °C for 1.5 h. The amount of H2S was controlled by using excess amount of HCl and changing the dosage of FeS. The results presented in Fig. 13 showed that the Cu precipitation rate increased rapidly when the sulfide-to-copper molar ratio increased from 1.6:1 to 2.4:1. Thereafter, it remained unchanged with further increase of sulfide-to-copper molar ratio to 2.8:1. The maximum Cu precipitation rate was 99.6%. This indicated that Cu was completely reacted at sulfide-to-copper molar ratio of 2.4:1 which was larger than the theoretical value of sulfide-to-copper molar ratio. A contributing factor was that a fraction of the H2S remained to the head space of the reaction apparatus. However, the precipitation rate of As was kept at around 0.1%. Hence, the sulfide-to-copper molar ratio of 2.4:1 was selected for the subsequent experiments.

Fig. 13 Effect of sulfide-to-copper molar ratio on precipitation ratios of Cu and As
3.3.2 Effect of reaction time
The effect of reaction time varying from 0.5 to 2.5 h on precipitation ratio was investigated at 25 °C with sulfide-to-copper molar ratio of 2.4:1. The precipitation rate curves are given in Fig. 14. It can be seen from Fig. 14 that the Cu precipitation rate increased with increase in the reaction time until 1.5 h. It remained constant at reaction time longer than 1.5 h. In contrast, the As precipitation rate remained to be constant at reaction time between 0.5 and 1.5 h and increased gradually with the increase of reaction time after 1.5 h. Therefore, a reaction time of 1.5 h was selected to guarantee the separation of Cu and As, for which the precipitation rates of Cu and As were 99.5% and 0.05%, respectively.
3.3.3 Effect of temperature
The effect of temperature in the range of 25-65 °C on precipitation rate was studied using sulfide-to-copper molar ratio of 2.4:1 and reaction time of 1.5 h, and the results are shown in Fig. 15. In the range of 25-65 °C, the precipitation rate of Cu was stable at about 99.6%, whereas the precipitation rate of As slightly increased from 0.01% to 0.31%. This indicated that elevated temperature was conducive to As precipitation, but had little effect on Cu precipitation. Hence, 25 °C was adjudged to be the optimum reaction temperature for selective precipitation of Cu.

Fig. 14 Effect of reaction time on precipitation rates of Cu and As

Fig. 15 Effect of temperature on precipitation rates of Cu and As
3.3.4 Optimum conditions
Based on the above results for the selective sulfide precipitation process, five sets of parallel experiments were carried out under optimal conditions of sulfide-to-copper molar ratio of 2.4:1, reaction time of 1.5 h and temperature of 25 °C. The results are shown in Fig. 16. As can be observed, the results of the five parallel experiments were similar. Under the optimal conditions, the average precipitation rates of Cu and As were 99.4% and 0.1%, for corresponding average Cu and As concentrations of (0.2±0.1) and (15.0±0.1) g/L in the leach solution, respectively. Therefore, Cu and As can be effectively separated.

Fig. 16 Results of repeated experiments under optimal conditions for selective sulfide precipitation of Cu
Figure 17 shows the XRD pattern of the precipitate, indicating that CuS was the dominant phase. The SEM image and EDS analysis are shown in Fig. 18. The particles occurred mostly as aggregates with loose microstructure. The sulfide- to-copper molar ratio was close to 1:1 and there was almost no As in the precipitates, which further indicated that Cu was successfully separated from leachate and recovered as CuS.

Fig. 17 XRD pattern of precipitates
4 Conclusions
(1) A new hydrometallurgical route consisting of oxidation acid leaching and selective sulfide precipitation was proposed for separation and recovery of Cu from Cu-As-bearing slime.
(2) The optimum conditions for oxidation acid leaching were determined to be initial H2SO4 concentration of 1.0 mol/L, L/S ratio of 10 mL/g, leaching temperature of 80 °C and leaching time of 4 h, under which average leaching rates of Cu and As were 95.2% and 97.6%, respectively. According to kinetic analysis using the Avrami model, the Cu and As leaching reactions were indicated to follow the mixed control with the apparent activation energies of 33.6 kJ/mol for Cu leaching and 35.1 kJ/mol for As leaching.
(3) By selective sulfide precipitation under optimal conditions of sulfide-to-copper ratio of 2.4:1, time of 1.5 h and temperature of 25 °C, 99.4% of Cu was successfully separated from leachate and recovered as CuS. The new process provides an alternative method for treating Cu-As-bearing residue in terms of recovering Cu as CuS which can be returned to copper smelting for Cu recycling. Besides, the high concentration of As can be used to produce scorodite to get stabilized.
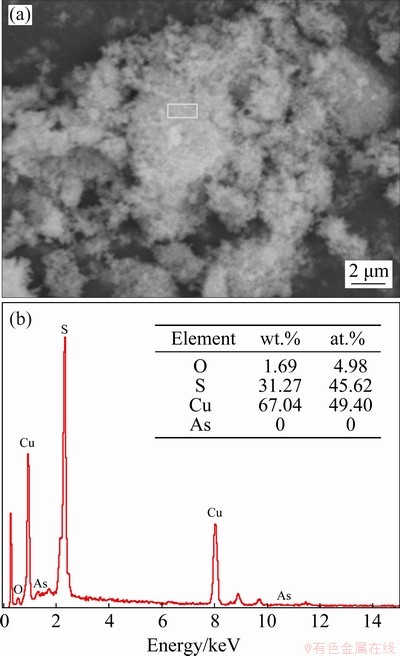
Fig. 18 SEM image (a) and corresponding EDS analysis results (b) of precipitates
Acknowledgments
The authors are grateful for the financial supports from the National Natural Science Foundation of China (51634010, 51904354), the National Science Fund for Distinguished Young Scholars of China (51825403), the National Key R&D Program of China (2018YFC1900306, 2019YFC1907405), and Key Research and Development Program of Hunan Province, China (2019SK2291).
References
[1] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, XIAO Lian-sheng. Identification of arsenato antimonates in copper anode slimes [J]. Hydrometallurgy, 2006, 84(3): 211-217.
[2] HAIT J, JANA R K, SANYAL S K. Mineralogical characteristics of copper electrorefining anode slime and its leached residues [J]. Industrial & Engineering Chemistry Research, 2004, 43(9): 2079-2087.
[3] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, WANG Ming-yu, TANG Fang. The role of arsenic in the homogeneous precipitation of As, Sb and Bi impurities in copper electrolyte [J]. Hydrometallurgy, 2011, 108(3-4): 199-204.
[4] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, WANG Ming-yu, XIAO Bing-rui, ZHANG Fan. Homogeneous precipitation of As, Sb and Bi impurities in copper electrolyte during electrorefining [J]. Hydrometallurgy, 2011, 105(3): 355-358.
[5] RANDHAWA N, HAIT J. Characteristics and processing of copper refinery anode slime [J]. Substainable and Economic Waste Management: Resource Recovery Techniques, 2019: 263.
[6] HOFFMANN J E. The purification of copper refinery electrolyte [J]. JOM, 2004, 56(7): 30-33.
[7] CHEN T T, DUTRIZAC J E. The mineralogy of copper electrorefining [J]. JOM, 1990, 42(8): 39-44.
[8] CHEN Yong-ming, LIU Nan-nan, YE Long-gang, XIONG Shan, YANG Sheng-hai. A cleaning process for the removal and stabilisation of arsenic from arsenic-rich lead anode slime [J]. Journal of Cleaner Production, 2018, 176: 26-35.
[9] YAO Li-wei, MIN Xiao-bo, XU Hui, KE Yong, WANG Yun-yan, LIN Zhang, LIANG Yan-jie, LIU De-gang, XU Qiu-jing, HE Yu-yang. Physicochemical and environmental properties of arsenic sulfide sludge from copper and lead-zinc smelter [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 1943-1955.
[10] MIN Xiao-bo, PENG Tian-yu, LI Yang-wen-jun, KE Yong, LIANG Yan-jie, HE Xing-yu. Stabilization of ferric arsenate sludge with mechanochemically prepared FeS2/Fe composites [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 1983-1992.
[11] CHEN T T, DUTRIZAC J E. Mineralogical characterization of anode slimes-9: The reaction of kidd creek anode slimes with various lixiviants [J]. Canadian Metallurgical Quarterly, 1993, 32: 267-279.
[12] FILIPPOU D, ST-GERMAIN P, GRAMMATIKOPOULOS T. Recovery of metal values from copper—arsenic minerals and other related resources [J]. Mineral Processing and Extractive Metallurgy Review, 2007, 28(4): 247-298.
[13] XU Hui, MIN Xiao-bo, WANG Yun-yan, KE Yong, YAO Li-wei, LIU De-gang, CHAI Li-yuan. Stabilization of arsenic sulfide sludge by hydrothermal treatment [J]. Hydrometallurgy, 2020, 191: 105229.
[14] KASHIWAKURA S, OHNO H, MATSUBAE-YOKOYAMA K, KUMAGAI Y, KUBO H, NAGASAKA T. Removal of arsenic in coal fly ash by acid washing process using dilute H2SO4 solvent [J]. Journal of Hazardous Materials, 2010, 181(1): 419-425.
[15] GUO Xue-yi, SHI Jing, YI Yu, TIAN Qing-hua, LI Dong. Separation and recovery of arsenic from arsenic-bearing dust [J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 2236-2242.
[16] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, ZHAO Zhong-wei, LIU Zhi-yong, ZENG Li. Removal of arsenic from Waelz zinc oxide using a mixed NaOH-Na2S leach [J]. Hydrometallurgy, 2011, 108(3): 165-170.
[17] MIHAJLOVIC I, STRBAC N, ZIVKOVIC Z, KOVACEVIC R, STEHERNIK M. A potential method for arsenic removal from copper concentrates [J]. Minerals Engineering, 2007, 20(1): 26-33.
[18] XU Zhi-feng, LI Qiang, NIE Hua-ping. Pressure leaching technique of smelter dust with high-copper and high-arsenic [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: s176-s181.
[19] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, ZHAO Zhong-wei, LIU Zhi-yong, ZENG Li, LI Li. Removal of arsenic from arsenate complex contained in secondary zinc oxide [J]. Hydrometallurgy, 2011, 109(3): 237-244.
[20] JIANG Guo-min, PENG Bing, CHAI Li-yuan, WANG Qing-wei, SHI Mei-qing, WANG Yun-yan, LIU Hui. Cascade sulfidation and separation of copper and arsenic from acidic wastewater via gas-liquid reaction [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(4): 925-931.
[21] KE Yong, SHEN Chen, MIN Xiao-bo, SHI Mei-qing, CHAI Li-yuan. Separation of Cu and As in Cu-As-containing filter cakes by Cu2+-assisted acid leaching [J]. Hydrometallurgy, 2017, 172: 45-50.
[22] LIU Cun-cheng, LIU Su-cheng, QIN Yuan-hang, MA Jia-yu, WU Zai-kun, ZHOU Jun-feng, LYU Ren-liang, WANG Cun-wen. The intensified leaching behavior of potassium from phosphorus-potassium associated ore in HCl-CaF2 system with surfactant: Part I Kinetics and modelling [J]. Separation and Purification Technology, 2019, 212: 89-100.
[23] REYES I A, PATINO F, FLORES M U, PANDIYAN T, CRUZ R, GUTIERREZ E J, REYES M, FLORES V H. Dissolution rates of jarosite-type compounds in H2SO4 medium: A kinetic analysis and its importance on the recovery of metal values from hydrometallurgical wastes [J]. Hydrometallurgy, 2017, 167: 16-29.
[24] DICKINSON C F, HEAL G R. Solid-liquid diffusion controlled rate equations [J]. Thermochimica Acta, 1999, 340-341: 89-103.
[25] OKUR H, TEKIN T, OZER A K, BAYRAMOGLU M. Effect of ultrasound on the dissolution of colemanite in H2SO4 [J]. Hydrometallurgy, 2002, 67(1-3): 79-86.
[26] GU Kun-hong, LI Wen-hua, HAN Jun-wei, LIU Wei, QIN Wen-qing, CAI Lian-bing. Arsenic removal from lead-zinc smelter ash by NaOH-H2O2 leaching [J]. Separation and Purification Technology, 2019, 209: 128-135.
史美清1,闵小波1,2,沈 忱1,柴立元1,2,柯 勇1,2,颜 旭1,2,梁彦杰1,2
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083
摘 要:开发从含铜砷的铜电解黑泥中分离和回收铜的湿法冶金新工艺。该工艺包括黑泥氧化酸浸和浸出液中选择性硫化沉铜两个步骤。研究各种工艺参数对铜和砷的浸出和沉淀的影响。在第一阶段中,最佳工艺条件为:初始H2SO4浓度为1.0 mol/L,液固比为10 mL/g,80 °C下连续浸出4 h。此条件下铜浸出率可达95.2%,砷浸出率为97.6%。同时,通过Avrami模型成功模拟氧化酸浸过程铜和砷的浸出动力学,发现铜和砷浸出反应的表观活化能分别为33.6和35.1 kJ/mol,表明该浸出过程主要受化学反应和扩散混合控制。在选择性硫化沉淀过程中,最佳工艺条件为:硫与铜摩尔比2.4:1、时间1.5 h、温度25 °C。此条件下99.4%的铜以CuS形式回收,而砷的沉淀率仅0.1%。
关键词:铜电解黑泥;氧化酸浸;选择性硫化沉淀;浸出动力学;铜回收
(Edited by Xiang-qun LI)
Corresponding author: Yong KE; Tel: +86-731-88830511; Fax: +86-731-88710171; E-mail: keyong000ke@csu.edu.cn
DOI: 10.1016/S1003-6326(21)65564-4
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press

