J. Cent. South Univ. Technol. (2011) 18: 966-971
DOI: 10.1007/s11771-011-0788-1
Effects of sintering temperature on microstructure and performance of Ti-based Ti-Mn alloy anodic material
HOU Yong-dan(侯永丹), JIANG Yao(江垚), LEI Ting(雷霆), HE Yue-hui(贺跃辉)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: A novel Ti-based Ti-Mn composite anode used for electrolytic manganese dioxide (EMD) fabrication was developed by a two-step heating manganizing technique. The effects of sintering temperature on the manganized microstructure and the performance of the composite anode were studied by scanning electron microscopy (SEM), mechanical properties tests at room temperature and electrochemical methods. The results show that the thickness of the diffusion layer increases with the increase of sintering temperature up to 1 100 °C; whereas, the surface Mn content increases and reaches the maximum at 1 000 °C and then decreases thereafter. Lower surface Mn content is beneficial for the enhanced corrosion resistance and lowered open cell voltage in electrolytic process. The new anode prepared under the optimized conditions has been applied in industry and exhibits superior economic benefits to conventional Ti anodic materials.
Key words: Ti-based Ti-Mn composite anode; electrolytic manganese dioxide (EMD); microstructure; electrochemical property; corrosion resistance property; mechanical properties
1 Introduction
Electrolytic manganese dioxide (EMD) is widely used as depolarizer in primary batteries such as Zn-Mn [1-5] and Li-Mn cells [6-8]. The quality of EMD product plays an important role in the discharge performance and storage life of batteries [9].
In the EMD industry, titanium is largely used as alternative anode electrode to conventional graphite, lead and its alloys for EMD production with respect to its high mechanical strength and good corrosion resistance [10]. Unfortunately, titanium anode can be easily passivated, depending on the current density (>70 A/m2), the electrolyte composition, the bath temperature, the surface condition and the service time. Finally, the passivated Ti needs to be activated either by sand blasting, chemical etching or cathode reduction [11]. Ti-Mn alloy [12] was also used as anodic material to overcome these limitations, which can withstand a higher current density of 300 A/m2 without obvious passivation [13]. Unfortunately, the brittleness of Ti-Mn alloy limited its commercial application in industry. In recent years, Ti-based Ti-Mn alloy was developed and widely used as anodic electrode in modern EMD industry. Basically, Ti-based Ti-Mn composite was prepared by surface modification through sintering. PREISLER et al [14] reported the fabrication of Ti-based Ti-Mn composite by sintering the Ti substrate coated with Mn powders in vacuum or in an inert environment at 800-1 150 °C for approximate 4.5 h. The thickness of the alloy layer was 100-300 ?m with surface Mn content more than 16% (mass fraction). Beijing Nonferrous Metal Research Institute [15] reported the sintering of Ti substrate coated with Ti-Mn mixed powder at high temperature (1 200 °C). The thickness of the as-prepared alloy layer was 250-340 ?m with surface Mn content more than 12%-14%. STEFFENS and BRUNE [16] developed the Ti-Mn electrode layers for EMD using vacuum plasma spray, which contained 30% Mn. Due to its high cost of preparation, it was proved unsuccessful in industry.
As enumerated above, Ti-based Ti-Mn composite anodic materials reveal many problems, such as large particle size, brittleness, cracks, easy deformation, and difficulty in removal of EMD products. A two-step heating manganizing technique for Ti-based Ti-Mn composite alloy fabrication was developed to solve some of the essential problems by sintering Ti substrate with a mixed coating containing Mn powder and additives. Effects of sintering temperature on the microstructure and performance of the as-obtained composite anode were studied.
2 Experimental
2.1 Preparation of Ti-based Ti-Mn composite anode
Commercially pure Ti substrate was cut into slices of 10 mm×10 mm×1.5 mm by a wire-electrode cutting equipment after initial sandblasting of the substrate surface, cleaned in ethanol in an ultrasonic bath, rinsed with distilled water and dried under an air stream with a standard atmospheric pressure. A mixture comprising of binder, inorganic solvent and Mn powder was coated on the Ti substrates at room temperature. The substrates were then sintered in a vacuum furnace according to a newly developed two-step heating manganizing process [17] including a pre-sintering treatment at a temperature above 700 °C and subsequent sintering at elevated temperatures of 950, 1 000 and 1 100 °C, respectively. Finally, residual Mn powder was cleaned with an acid solution.
2.2 Microstructure and performance characterization
For the characterization of the sample morphology and composition, a field-emission SEM NANO NOVA230 equipped with energy dispersive X-ray (EDX) analyzer was used. The roughness was identified with Vision (America). All electrochemical measurements were performed in a conventional three-electrode cell, using a large copper plate as the counter electrode and a saturated calomel electrode as the reference electrode. All experimental potential values were reported versus saturated calomel electrode. The solution used in the experiment was 97.3 g/L MnSO4 + 42.6 g/L H2SO4 from EMD industry. The bath temperature was (96±1) °C. Prior to all electrochemical measurements, the specimens were initially activated at -1 V for 120 s to remove air-formed oxides from the surface and then immersed in the solutions until a stable corrosion potential was reached. All the electrochemical tests were carried out using CHI660C electrochemical measurement system. Potentiodynamic polarization curves were acquired at a scanning rate of 1 mV/s in MnSO4 electrolyte solution. The corrosion current and the corrosion rate were estimated under the specified range. The direct constant current density for electrolyzing was 100 A/m2 for 30 min. Mechanical properties were investigated with Instron 3369 mechanical testing machine (America).
3 Results and discussion
3.1 Microstructure of Ti-based Ti-Mn composite anode
Figure 1 shows the SEM microstructure of the composite anodes prepared at different sintering temperatures, and the contents of manganese at the surface is displayed in Table 1. As observed in the inset high resolution images, the surface of the composite anodes sintered at 1 100 °C is smooth. In comparison, the composite specimens sintered at 950 °C and 1 000 °C exhibit an extended rugged surface, indicating an enlarged effective anode surface area. Note that no significant cracks are visible on the surface of specimens sintered at 950 °C and 1 100 °C, but cracks can be observed microscopically for specimen sintered at 1 000 °C. It may be resulted from the shrinkage of Mn powders during the sintering process. When sintering temperature as high as 1 100 °C is applied, liquid phase of Mn may flow into and fill the cracks, resulting in the formation of dense structure. As a result, the specimen sintered at 1 100 °C exhibits smooth and dense surface.
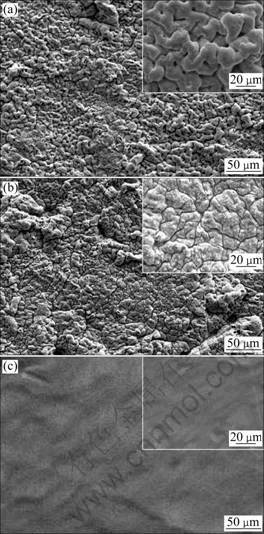
Fig.1 SEM images of Ti-based Ti-Mn composite anodes prepared at different temperatures: (a) 950 °C; (b) 1 000 °C; (c) 1 100 °C
Table 1 Surface Mn contents of composite anodes

SANTOS and DYMENT [18] reported that the diffusion coefficient and active energy of Ti as well as the diffusion coefficient of Mn diffusing into Ti matrix increased with the increase of Mn content. However, there exists a critical temperature, which is about 1 040- 1 100 °C. Basically, Mn has high diffusion rate in Ti substrate and the diffusivity of Ti decreases with Mn addition at sintering temperature lower than the critical temperature. On the contrary, the diffusivity of Ti increases with Mn addition at sintering temperature higher than the critical temperature. As a result, the surface Mn content of the specimens increases with the increase of sintering temperature up to 1 000 °C, and then decreases dramatically at 1 100 °C as shown in Table 1, which is consistent with the report in Ref.[18].
The roughness of the composite anodes was measured to be 3.61, 3.37 and 2.52 ?m, corresponding to sintering temperature of 950, 1 000, and 1 100 °C, respectively, which is in good agreement with the SEM observation. Apparently, the coatings produced by the new method provide a dense structure, which may act as a physical shield to improve the corrosion resistance.
The cross-section BED images and line scanning maps of the composite anodes are shown in Fig.2, which depicts a diffusion depth of Mn (light areas) in Ti (dark areas) substrate. Apparently, the manganized layer of the Ti-based Ti-Mn composite consists mainly of two layers, which are Mn-rich layer close to the surface and acicular Mn-diffusion layer close to the substrate. The contents of Ti and Mn in the manganized layer exhibit gradient changes. Moreover, there is no obvious interface between the coating and substrate, indicative of good metallurgy bonding between them. XRD result shows that the Mn-rich layer is TiMn2, and the acicular Mn layer is α+β alloy, which coincide with the previously reported results [19-20]. The BED image of the new anode sintered at 950 °C followed by water quenching instead of furnace cooling is shown in Fig.3, which reveals three clear gradient layers corresponding to TiMn2 intermetallic, Ti-Mn solid solution and Ti substrate (from surface to the substrate). This result reveals that α phase is formed as a precipitated phase from super-saturated β solid solution along the grain boundary during the furnace cooling process, and this leads to the formation of α+β alloy layer.
The layer thickness and surface element analysis of the composite anodes are given in Table 2. It is observed from Table 2 that the thickness increases gradually when the sintering temperature is below 1 000 °C and rises sharply at 1 100 °C. Interestingly, a coating layer with the highest surface Mn content is formed on Ti substrate after sintering at 1 000 °C. The grain size of the composite anodes obtained from XRD is 40.9, 52.9 and 63.1 nm for specimens sintered at 950, 1 000 and 1 100 °C, respectively. It can be seen that the grain size increases with the increase of the sintering temperature.

Fig.2 BED images and line scanning maps of composite anodes prepared at different temperatures: (a) 950 °C; (b) 1 000 °C; (c) 1 100 °C

Fig.3 BED image of composite anode sintered at 950 °C followed by water quenching
Table 2 Layer thicknesses and surface element contents of composite anodes

3.2 Electrochemical behaviour of Ti-based Ti-Mn composite anode
The corrosion resistance of the Ti-based Ti-Mn composite anodic electrodes was determined in MnSO4 solution using potentiodynamic polarization tests, as shown in Fig.4. The corrosion potential (φcorr) and corrosion current density (Jcorr) were derived directly from the polarization curves by Tafel region extrapolation. The results of potentiodynamic polarization are summarized in Table 3. The positive shift of corrosion potentials accompanied by the decrease of corrosion currents indicate that the tendency to corrosion of the composite anodic electrode decreases. The polarization test results lead directly to the conclusion that the composite anode sintered at 1 100 °C exhibits significantly improved corrosion resistance.

Fig.4 Tafel curves of composite anodes in industrial electrolyte
Table 3 Electrochemical corrosion parameters from Tafel curves of composite anodes in industrial electrolyte

The galvanostatic polarisation curves of the composite anodes under a direct constant current in the industrial electrolyte are given in Fig.5. It is observed that the galvanostatic polarisation curves of the composite anode sintered at 950 and 1 100 °C become stable after 800 and 1 180 s of electrolysis, respectively; and the curve at 1 000 °C decreases linearly after 400 s. The time to reach the stability is 280, 131 and 17 s for 950, 1 000 and 1 100 °C, respectively.

Fig.5 Electrolytic curves of composite anodes under constant current in industrial electrolyte
In EMD industry, the operating current is increased gradually up to the electrolysis current in 15-20 min, and the corresponding potential is denoted as the open cell voltage. Accordingly, the open cell voltage of the galvanostatic polarisation curve in this experiment is obtained when the potential is stable. As shown in Fig.5, the bath voltages of the composite anode sintered at 950, 1 000 and 1 100 °C are approximately 1.253 1, 1.680 8, and 0.996 4 V, respectively. Apparently, the surface Mn content of composite anode has effect on the open cell voltage which drops with the decrease of Mn content at different sintering temperatures.
The fabrication of EMD by electrolysis process can be divided into three processes, which are generation, breakdown, and compactness formation steps. In the initial electrolysis process, Mn2+ is oxidized by losing electrons, leading to the formation of MnO2 as shown in the following formula:
 (1)
(1)
As the electrolysis progresses, the oxidation of Mn2+ continues and thus the thickness of MnO2 coating layer increases. Due to the high dielectric constant of MnO2 layer, the MnO2 layer exhibites passivation gradually. This leads to the increase of electric resistance of the anode electrode and thus the bath voltage increases. When the voltage is high enough, the breakdown of MnO2 layer occurs, which is a driving force for continued Mn2+ oxidation and then a new balance is built up gradually, resulting in the formation of compact MnO2 layer. Hence, the larger the real surface area, the more the electric charges and longer time would be needed to form the equivalent thick passivation layer. As a result, the time when the breakdown occurs would delay for an anode with large surface area, as shown in Fig.5. Therefore, it is reasonable to predict that the sequence of the real surface area of the composite anode from small to large follows 1 100, 1 000 and 950 °C. This prediction is in good conformity with the roughness measurements in Section 3.1.
3.3 Mechanical properties of composite anodes at room temperature
Table 4 lists the mechanical properties of the composite anodes sintered at 950, 1 000 and 1 100 °C, respectively. The elongation of the composite anodes after fracture decreases with the increase of sintering temperature. In comparison, the tensile strength increases with increasing the sintering temperature.
As discussed in Section 3.1, the manganized layer of the Ti-based Ti-Mn composite consists mainly of two layers, which are intermetallic TiMn2 layer close to the surface and Ti-Mn solid solution layer close to the substrate. The surface intermetallic compound of TiMn2 is detrimental to the toughness of the composite anode and Ti-Mn alloy acts as reinforcement due to solid solution strengthening. Ti matrix is known to have a high toughness, and the particle size has significant influence on the impact toughness. Small particle size can improve the toughness. As discussed earlier, with the increase of the sintering temperature, the manganized layer of the Ti-based Ti-Mn composite becomes thicker, in other words, the amounts of TiMn2 and Ti-Mn alloy increase. Meanwhile, the particle size also increases with increasing the sintering temperature. As a result, the integrated contribution leads to the decrease of toughness and the increase of strength for the composite anodes. Accordingly, it is important to optimize the sintering process to control the manganized layer thickness and particle growth to achieve a good toughness and strength to withstand the impact when EMD products are mechanically removed. In this experiment, the Ti-based Ti-Mn composite anode sintered at 950 °C appears higher toughness.
3.4 Application in industry
Conventional Ti-based Ti-Mn composite anode [21] widely used in industry to date has a 3D porous structure on the top of the Ti substrate, exhibiting a rough surface and leading to a good adhesive attraction of EMD products, but the problem of this structure is the decrease of compactness leading to poor corrosion resistance, short lifespan and difficulty in the removal of EMD products. By contrast, the composite anode prepared by this new method has a homogeneous and compact coating surface, leading to a good corrosion resistance and a rough surface, which makes the attachment and the removal of the EMD products easier.
The new Ti-based Ti-Mn composite anode sintered at 950 °C reveals lower electrolysis voltage and best mechanical properties, and therefore is used in EMD fabrication in industry. Besides, this two-step heating manganizing technique is also used to renew the used Ti anode, and the results are summarized in Table 5. It could be seen that the new Ti-based Ti-Mn composite anode may extend the production period from 5.5 to 9.5 d. After three cycles of production, the bath voltage keeps stable, indicative of a good stability of the anode material in the practical use. Meanwhile, lower bath voltage under a high current density (108 A/m2) could enhance the current efficiency and the quality of the EMD products. Accordingly, the new Ti-based Ti-Mn composite anode material could improve both the quantity and the quality of the EMD products. Moreover, compared with the data obtained from the anode [22] shown in Table 5, the renewed anode reveals similarly good electrolytic properties. Most importantly, the application of the two-step heating manganizing technique in the renewal of used Ti anode largely used so far in EMD industry is a good solution to the difficulty of removing EMD product and cracks formation on the anode materials in mechanical removal process.
Table 4 Mechanical properties of composite anodes at room temperature

Table 5 Production data of Ti-based Ti-Mn composite anodes
4 Conclusions
1) A novel Ti-based Ti-Mn composite anode was developed by a two-step heating manganizing technique.
2) There exists a critical temperature for diffusivity of Ti with the addition of Mn. The thicknesses of the Mn-rich layer are 45-50, 70-80 and 142-155 μm with corresponding surface Mn contents of 29%-34%, 38%- 43% and 23%-27% for composite anodes sintered at 950 , 1 000 and 1 100 °C, respectively.
3) The Ti-based Ti-Mn composite anodes sintered at 950 and 1 000 °C exhibit an enlarged effective anodic surface area due to the extended rugged surface, while that sintered at 1 100 °C shows a smooth surface morphology.
4) The Ti-based Ti-Mn composite anode sintered at 950 °C shows advantages of lower electrolysis voltage and better mechanical properties over the conventional Ti anode, and is successfully used in EMD fabrication in industry.
5) The two-step heating manganizing technique provides a simple way for the renewal of used Ti anode largely employed currently.
References
[1] NAN Jun-min, HAN Dong-mei, CUI Ming, YANG Min-jie, PAN Lin-mao. Recycling spent zinc manganese dioxide batteries through synthesizing Zn-Mn ferrite magnetic materials[J].Journal of Hazardous Materials, 2006, 133(1/3): 257-261.
[2] MINAKSHI M, SINGH P, ISSA T B, THURGATE S, DE MARCO R. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery (Part III): Electrochemical behavior of γ-MnO2 in aqueous lithium hydroxide electrolyte [J]. Journal of Power Sources, 2006, 153(1): 165-169.
[3] GHAEMI M, GHOLAMI A, MOGHADDAM R B. A study around the improvement of electrochemical activity of MnO2 as cathodic material in alkaline batteries [J].Electrochimica Acta, 2008, 53(8): 3250-3256.
[4] AARON P MALLOY, SCOTT W DONNE. Chronoamperometric characterization of manganese dioxide discharge in alkaline electrolytes [J]. Journal of Electroanalytical Chemistry, 2008, 621(1): 83-90.
[5] DANIEL-IVAD J. Secondary batteries-zinc systems/zinc-manganese [M]. Encyclopaedia of Electrochemical Power Sources, 2009: 497-512.
[6] JOHNSON C. Development and utility of manganese oxides as cathodes in lithium batteries [J]. Journal of Power Sources, 2007, 165(2): 559-565.
[7] LIU Yun-jian, LI Xin-hai, GUO Hua-jun, WANG Zhi-xing, HU Qi-yang, PENG Wen-jie. Overcharge performance of LiMn2O4/graphite battery with large capacity [J]. Journal of Central South University of Technology, 2009, 16(5): 763-767.
[8] NISHIO K. Primary batteries-nonaqueous systems/Lithium- Manganese dioxide [C]. Encyclopaedia of Electrochemical Power Sources, 2009: 83-92.
[9] LEE S, CHOI B, HAMASUNA N, FUSHIMI C, TSUTSUMI A. Characterization of MnO2 positive electrode for fuel cell/battery (FCB) [J].Journal of Power Sources, 2008, 181(1): 177-181.
[10] PANG Jing, HUANG Song-tao, HU Yong-hai. Application and development of anode materials used in EMD production [J]. Rare Metals, 2001, 25(5): 369-373.
[11] PREISER E. Material problems encountered in anodic MnO2 deposition [J]. Journal of Applied Electrochemistry, 1989, 19(4): 559-565.
[12] ATLADZE R I. Anode: SU 484 893 [P]. 1973-05-14.
[13] AGLADZE R I, ZAUTASHVILI L A, VANIDZE K S H. The possibility of using Ti-Mn alloys as anode material in electro- deposition of MnO2 [J]. Elektrokhimiya, 1980, 16(12): 1779-1785.
[14] PREISER E, DEBRODT N, LIEBEROTH W. Activated metal anodes and a process for making them: US 4 589 960 [P]. 1986-05- 20.
[15] Beijing Nonferrous Metal Research Institute. Composite anode for electrolysis: CN87216402U [P]. 1987-12-21.
[16] STEFFENS H D, BRUNE M. Vacuum-plasma-sprayed titanium- manganese electrode layers for MnO2 deposition [J]. Journal of Thermal Spray Technology, 1995, 4(1): 85-88.
[17] HE Yue-hui, XIAO Yi-feng, CHEN Yu-zhang, LI Tong-qing, LI Jian, LI Qing. Preparation method for coating titanium anode for the production of electrolytic manganese dioxide: CN101603180A [P]. 2009-06-09.
[18] SANTOS E, DYMENT F. Solvent and solute diffusion in b.c.c. Ti-Co and Ti-Mn alloys [J]. Philosophical Magazine, 1975, 31(4): 809-827.
[19] HU Xian-feng, YU Zheng, YANG Sheng-shu. Determine for surface phases of Ti-base alloys anode by X-ray diffraction [J]. Journal of Guizhou University: Natural Science, 1999, 16(1): 35-40. (in Chinese)
[20] KUNDU S, GHOSH M, CHATTERJEE S. Diffusion bonding of commercially pure titanium and 17-4 precipitation hardening stainless steel [J]. Materials Science and Engineering A, 2006, 428: 18-23.
[21] HUANG Song-tao, DONG Jun-qing, HU Yong-hai, HE Fen. Development of high efficient composite anodes [J]. Rare Metals, 1999, 5(1): 02-08.
[22] WEI Yuan-xian, ZHOU Ling-feng, ZHAI Jun-ying, DONG Jun-qing. Application of Ti-Mn alloy coated electrodes in Xia Tian Manganese Mine [J]. China’s Manganese Industry, 1994, 12(6): 42-45. (in Chinese)
(Edited by HE Yun-bin)
Foundation item: Projects(20476106, 50721003 and 20636020) supported by the National Natural Science Foundation of China; Project(50825102) supported by the National Natural Science Funds for Distinguished Young Scholar of China; Project(2006AA03Z511) supported by the National High Technology Research and Development Program of China; Project supported by the 111 Program of Chinese Ministry of Education
Received date: 2010-12-03; Accepted date: 2011-02-23
Corresponding author: JIANG Yao, PhD; Tel: +86-731-88877391; E-mail: jiangyao@mail.csu.edu.cn