脉冲电流处理对一种镍基耐蚀合金腐蚀行为的影响
来源期刊:中国有色金属学报(英文版)2011年第9期
论文作者:刘杨 王磊 刘红艳 张北江 赵光普
文章页码:1970 - 1975
关键词:镍基合金;脉冲电流;不连续析出;晶间腐蚀
Key words:nickel base alloy; electropulsing; discontinuous precipitation; intergranular corrosion
摘 要:将脉冲电流处理应用于一种镍基耐蚀合金的时效过程,研究脉冲电流对合金显微组织及耐蚀性的影响规律及机理。结果表明,脉冲电流处理后,合金在力学性能基本保持不变的前提下,其耐晶间腐蚀性能得到显著提高。脉冲电流能够显著提高合金元素的界面扩散速度,其界面扩散速度远快于体扩散速度,晶界M23C6型碳化物呈典型的不连续析出转变特征。与常规时效处理相比,脉冲电流处理后晶界碳化物的不连续析出转变,使晶界位置的合金元素的贫化程度大幅度降低,在保证合金力学性能的前提下,显著改善合金的耐晶间腐蚀能力。
Abstract: Electropulsing treatment (EPT) was performed on a nickel base corrosion resistant alloy during aging. The effect of EPT on the microstructure and corrosion resistance of the alloy and the mechanisms were investigated. The results show that the intergranular corrosion resistance can be improved substantially without the degradation of mechanical properties of the alloy by EPT. The EPT has an effect of enhancing the interface diffusion rate of the alloying element, which is higher than the body diffusion rate. And thus discontinuous precipitation of M23C6 type carbides appears at the grain boundary in the alloy by EPT, which decreases the depletion extent of the alloying elements at the grain boundary substantially. As a result, the intergranular corrosion resistance of the alloy can be improved by the EPT without any degradation of mechanical properties.

LIU Yang1, WANG Lei1, LIU Hong-yan1, ZHANG Bei-jiang2, ZHAO Guang-pu2
1. Key Lab for Anisotropy and Texture of Materials, Northeastern University, Shenyang 110819, China;
2. Department of High-temperature Materials, Central Iron and Steel Research Institute, Beijing 100081, China
Received 30 October 2010; accepted 27 May 2011
Abstract: Electropulsing treatment (EPT) was performed on a nickel base corrosion resistant alloy during aging. The effect of EPT on the microstructure and corrosion resistance of the alloy and the mechanisms were investigated. The results show that the intergranular corrosion resistance can be improved substantially without the degradation of mechanical properties of the alloy by EPT. The EPT has an effect of enhancing the interface diffusion rate of the alloying element, which is higher than the body diffusion rate. And thus discontinuous precipitation of M23C6 type carbides appears at the grain boundary in the alloy by EPT, which decreases the depletion extent of the alloying elements at the grain boundary substantially. As a result, the intergranular corrosion resistance of the alloy can be improved by the EPT without any degradation of mechanical properties.
Key words: nickel base alloy; electropulsing; discontinuous precipitation; intergranular corrosion
1 Introduction
With the rapid development of aerospace industry, the alloying degree of superalloy is increasing and the working condition is going more and more severe [1-2]. Besides the excellent properties including the high temperature strength and plasticity, the oxidation resistance and corrosion resistance are also required [3-4]. Especially when the number and size of the grain boundary carbides change after long-term service, the grain boundary becomes weak under the specific corrosion conditions [5-6], which directly affects the safe service.
Electropulsing treatment (EPT) as a new method for the material processing has been widely noticed recently. The main researches of EPT are concentrated on refining solidification structure of metallic materials, effect of electro plasticity, and promoting the crystallization of amorphous materials [7-9]. While as for the superalloy used under severe working conditions, the microstructure evolution in the alloy by EPT and the effect of EPT on the properties of the alloy have not yet been reported. The present study aimed at performing EPT on a nickel base corrosion resistant alloy with the emphasis on the effects of EPT on microstructure and corrosion resistance of the alloy. And the mechanisms were also discussed.
2 Experimental
The alloy was vacuum induction melted and electroslag remelted, then rolled into plate with a thickness of 4 mm. The chemical compositions (mass fraction, %) of the alloy are: C 0.1, Si 0.65, Mn 0.40, P 0.01, S 0.01, Cr 25.60, W 14.50, Mo 1.45, Fe 3.95, Al 0.40, Ti 0.65 and Ni balance. After being solution treated at 1 150 °C for 5 min followed by air cooling, the plate was machined along the rolling direction into specimens of 0.5 mm×3 mm×100 mm for EPT.
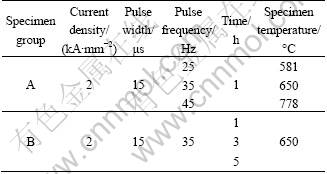
The specimens were electropulsing treated by a HPC-5 type EPT equipment. The EPT conditions were as follows: current density of 2 kA/mm2, pulse width of 15 μs for 1 h at pulse frequency of 25, 35 and 45 Hz, respectively (named A group), and current density of 2 kA/mm2, pulse width of 15 μs, frequency of 35 Hz for 1, 3 and 5 h, respectively (named B group). The specimen temperature was measured by an infrared temperature meter and a K-type thermocouple soldered onto the specimen surface. For comparison, the normal aging treatment was also performed on the specimens with the same process just without EPT. The conditions of EPT and the corresponding temperatures of the specimens are listed in Table 1.
Table 1 Conditions of EPT and corresponding real temperature of specimens
The corrosion test was carried out based on the Standard GB/T 15260—94. The corrodent was a solution of CuSO4 (16 g), HCl (80 mL) and C2H5OH (20 mL) and the etching time was 72 h. The results presented were an average of three specimens.
The mechanical properties of the specimens were examined by MTS 810 type material test system with a force sensor of 0-5 kN. The microstructure evolution, surface morphology of the alloy after corrosion were observed on an optical microscope (OLYMPUS GX71), laser scanning confocal microscope (OLYMPUS OLS 3100) and field emission scanning electron microscope (JEOL 7001).
3 Results
3.1 Effect of EPT on corrosion resistance of alloy
The normal aging treatment was carried out at 800 °C for 12 h followed by air cooling to satisfy the strength requirements for the alloy during service. The corrosion resistance and mechanical property of the specimen by EPT were compared with the normal aging one. The variations of corrosion rate and yield strength of the alloy by EPT are shown in Fig. 1. It can be seen that the corrosion rate of the alloy by EPT increased but was still less than that of the alloy by normal aging.
The yield strength increased substantially with increasing pulse frequency and treatment time. The yield strength of the alloy by EPT with pulse frequency of 45 Hz in group A and that of the alloy by EPT for 5 h in group B increased by 13.24% and 14.05% compared with that of the alloy by aging, respectively. But there is no obvious change in fracture elongation of the alloy by EPT under different conditions compared with that of the alloy by aging. It is indicated that the electropulse has a remarkable effect on the thermodynamics and kinetics of precipitation of the alloy. At a low temperature, there is a rapid precipitation of the strengthening phases by effect of electropulse, so that the yield strength of the alloy increases obviously.
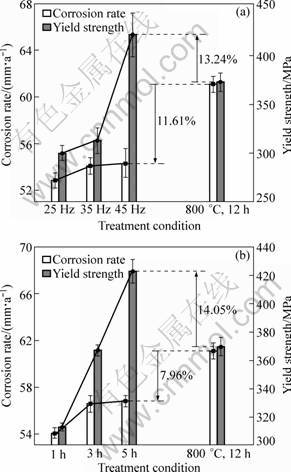
Fig. 1 Effect of pulse frequency in group A (a) and treatment time in group B (b) on corrosion rate and yield strength of alloy by EPT
It is worth noting that the increment of the corrosion rate of the alloy by EPT is much lower than that of the yield strength. It can be concluded that the corrosion resistance of the alloy increased obviously with the yield strength and the plasticity of the alloy basically did not change. By comparing with normal aging, the yield strength of the specimen by EPT increased by 13.24% and the corrosion rate decreased by 11.61% under the condition of 2 kA/mm2, 15 μs, 45 Hz for 1 h, and the yield strength increased by 14.05% and the corrosion rate decreased by 7.96% under the condition of 2 kA/mm2, 15 μs, 35 Hz for 5 h. In other words, the corrosion rate of the alloy can be improved by EPT without degradation of mechanical properties, which is very important for the application of the alloy.
3.2 Morphology of corrosion surface of alloy by EPT
Figure 2 shows the surface morphologies of the alloys after corrosion under different conditions. There are a large amount of net-like corrosion ditches on the surface of the alloy by aging after corrosion, which shows the typical intergranular corrosion morphology. It indicates that the grain boundaries of the alloy by aging are corroded preferentially in the present study, and displays typical intergranular corrosion.
Nevertheless, the corrosion surface fluctuation of the alloy by EPT is similar to that by solution treatment and the surface displays the typical uniform corrosion. With increasing the treating time of EPT, the surface fluctuation increases and there is no obvious change of the microstructure in the alloy. It is indicated that both the intergranular corrosion and the corrosion rate can be reduced by the EPT compared with aging.
4 Discussion
4.1 Discontinuous precipitation of grain boundary carbide by EPT
In the austenitic stainless steel and corrosion resistant alloy, the precipitation of M23C6 type grain boundary carbides can result in a depletion of the nearing Cr element, which is the main cause of intergranular corrosion [10]. As a result, the carbides will precipitate and grow at the grain boundary and the obvious intergranular corrosion appears under specific corrosion conditions.
After EPT, the yield strength of the alloy increases and the yield strength is higher than that of the alloy by aging even when being treated at a low temperature for short treatment time. In addition, it can be seen from the results of the chemical compositions and the phase analysis that there is no other phase transformation except for the dissolving and precipitation of carbides in the alloy [11]. It is indicated that the EPT does not inhibit the precipitation of the carbides but changes the morphology and distribution of precipitation of the carbides. Consequently, EPT improves the distribution of elements at the grain boundary, inhibits the intergranular corrosion and decreases the corrosion rate substantially with assurance of strengthening the substrate.
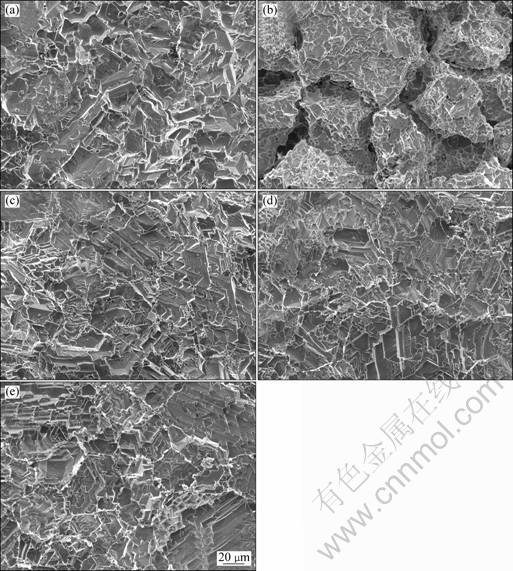
Fig. 2 SEM images showing surface morphologies of alloys after corrosion by solution treatment (a), by aging at 800 °C for 12 h (b), and by EPT under condition of 2 kA/mm2, 15 μs and 35 Hz for 1 h (c), 3 h (d), and 5 h (e), respectively
Researches on the microstructure evolution by EPT showed that the electropulse has a promoting effect on the element diffusion in the alloy and thus changes the thermodynamics and kinetics of precipitation of M23C6 type grain boundary carbides [12]. EPT not only speeds the precipitation of grain boundary carbides but also decreases the peak temperature of precipitation. And after EPT, the grain boundary carbides in the alloy can precipitate at a lower temperature rapidly. As a result, the yield strength increases rapidly in the alloy by EPT with increasing treatment time even at a lower temperature for a shorter time compared with aging.
Figure 3 shows the microstructures of the alloy under different conditions. It can be seen that the M23C6 type carbides precipitate and grow at the grain boundaries of the alloy by aging. In contrast with this, the percentage of the grain boundary carbides of the alloy by EPT with different pulse frequency increases substantially and it is more than that by aging. And some carbides precipitate at grain boundary in cellular structure with increasing pulse frequency. Most of the cellular structure grows from the grain boundary to the inside of the grain and little grow towards the both side grains along the grain boundary. The typical discontinuous precipitation of the carbides appears [13].
It was reported that there is a strong impacting force of high-rate drift electrons on atoms during the passing of the electropulse [14]. The atomic thermal vibrations will be promoted by the periodic impact of high frequency, and then it leads the atoms up to high energy state. The minimum energy for the atom-bonds breaking with the neighbor atoms and jumping over energy barrier decreased. As a result, the activation energy for atom diffusion decreased with decreasing atomic transition energy barrier (ΔEe<ΔE).
For the diffusion in a metallic material, two dominative mechanisms are possible: interstitial diffusion and vacancy diffusion. Based on the crystal structure of M23C6 type carbide and the atom radii of Cr and Ni, it is clear that Cr locates in the lattice sites of γ phase and diffuses into the austenitic matrix by vacancy diffusion. The activation energy of diffusion includes two parts: activation energy for atomic transition ΔE and vacancy formation energy ΔEv. For an atom to be moved, there must be an empty adjacent site and the atom must have enough energy to break bonds with the neighbor atoms. EPT is a rapid heating process and usually the energy provided by such an external resource transfers into Joule heating. It was recognized that in metallic materials, dissipation of electronic excitation into heat is very fast and the rate of temperature rising can achieve an order of 106 °C/s [15]. Consequently, nonsynchronous change of temperature rising before dynamic thermal stress can be formed [16]. A mass of supersaturation point defects will be formed by the transient thermal stress, and therefore the vacancy density is increased. The atom transition frequency is proportional to the vacancy density by vacancy diffusion mechanism [17]. Consequently, increasing vacancy density is beneficial to the diffusion rate of atoms. It indicates that both ΔE and ΔEv will be decreased and the atom diffusion can be promoted by EPT. And thus the rate of interface diffusion of alloying element will be higher than that of body diffusion rate. Consequently, it appears to be a rapid discontinuous precipitation of the carbides at the grain boundary of the alloy by EPT [18].
4.2 Mechanisms of improvement of corrosion resistance of alloy by EPT
Figure 4 shows the variations of corrosion microstructure at the grain boundary of the alloy with the pulse frequency of EPT. It can be seen that there are obvious intergranular corrosion ditches at the grain boundary carbides in the alloy by aging. The corrosion ditches become deep into the alloy along the two sides of the carbides. It can be observed that the net-like carbides remain in the center of corrosion ditches (as indicated by black arrows in Fig. 4(a)).

Fig. 3 OM observation showing microstructures of alloy after solution treatment (a), after aging at 800 °C for 12 h (b), and after EPT under condition of 2 kA/mm2, 15 μs, with pulse frequency of 35 Hz (c) and 45 Hz (d) for 1 h, respectively
However, after EPT, the percentage of grain boundary carbides increases substantially with increasing pulse frequency and treatment time. And there is no obvious intergranular corrosion and no corrosion ditch in the alloy by EPT even at the front of the cellular structure with bigger size (as indicated by white arrows in Figs. 4(c) and (d)). It can be concluded that the discontinuous precipitation of grain boundary carbides in the alloy by EPT can improve the distribution of alloying element at the grain boundary and inhibit the intergranular corrosion behavior of the alloy.
Figure 5(a) shows the schematic illustration of depletion of the solute atoms near the grain boundary induced by precipitation of carbides by aging. Discontinuous precipitation is a phase transformation, of which the interface diffusion rate is much higher than the body diffusion rate of the solute atoms in the alloy [19]. The solute atoms diffuse rapidly at the interface of the cellular structure front to the precipitates and there is no solute depletion zone in the alloy due to the body diffusion of the solute atoms near the interface of cellular structure of discontinuous precipitation, which is different from the alloy by aging, as shown in Figs. 5(b) and (c). In other words, the alloying element needed for precipitation of carbides mainly originate from the diffuse flow of elements at the interface of the cellular structure front. The depletion zone cannot be formed and the intergranular corrosion hardly occurs in the alloy, which is the main reason for improving corrosion resistance of the alloy by EPT.
5 Conclusions
1) The intergranular corrosion resistance of the alloy can be improved by EPT without the degradation of mechanical properties. Compared with the alloy by aging at 800 °C for 12 h, the corrosion rate of the alloy by EPT decreased by 11.61% (under the conditions of 2 kA/mm2, 15 μs, 45 Hz, 1 h) and 7.96% (under the conditions of 2 kA/mm2, 15 μs, 35 Hz, 5 h), respectively.
2) The interface diffusion rate of the alloying element is higher than the body diffusion rate in the alloy by EPT. It appears to be a discontinuous precipitation of M23C6 type grain boundary carbides, which is the main reason for decreasing the depletion extent of the alloying elements at the grain boundary and improving the intergranular corrosion resistance of the alloy by EPT.
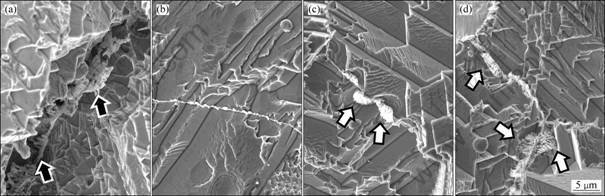
Fig. 4 SEM images showing microstructures at grain boundary of alloy after corrosion by aging (a), by EPT under condition of 2 kA/mm2, 15 μs with pulse frequency of 25 Hz (b), 35 Hz (c), and 45 Hz (d) for 1 h, respectively
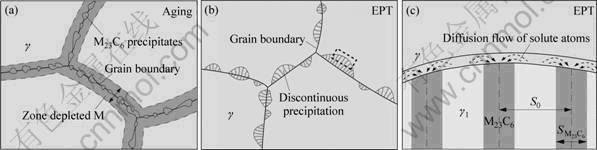
Fig. 5 Schematic illustration of mechanisms of improvement of corrosion resistance of alloy by EPT: (a) Depletion of alloying element near grain boundary induced by precipitation of M23C6 type carbides by aging; (b) Discontinuous precipitation of carbides at grain boundary by EPT; (c) Interface diffusion features of discontinuous precipitation (S: lamellar spacing)
[1] SHARQHI M R, ASQARI S. The influence of thermal exposure on the g?-precipitates characteristics and tensile behavior of superalloy IN-738LC [J]. Journal of Materials Processing Technology, 2004, 147(3): 343-350.
[2] XIE Xi-shan, DONG Jian-xin, FU Shu-hong, ZHANG Mai-cang. Research and development of γ? and γ? strengthened Ni-Fe base superalloy GH4169 [J]. Acta Metallurgica Sinica, 2010, 46(11): 1289-1302. (in Chinese)
[3] BAI C Y. Effects of electrical discharge surface modification of superalloy Haynes 230 with aluminum and molybdenum on oxidation behavior [J]. Corrosion Science, 2007, 49(10): 3889-3904.
[4] HAN En-hou, WANG Jian-qiu, WU Xin-qiang, KE Wei. Corrosion mechanisms of stainless steel and nickel base alloys in high temperature high pressure water [J]. Acta Metallurgica Sinica, 2010, 46(11): 1379-1390. (in Chinese)
[5] GORDON A P, TREXLER M D, NEU R W, NEU R W, SANDERS T J, MCDOWELL D L. Corrosion kinetics of a directionally solidified Ni-base superalloy [J]. Acta Materialia, 2007, 55(10): 3375-3385.
[6] CHEN Chang-feng, JIANG Rui-jing, ZHANG Guo-an, ZHENG Shu-qi, GE Lei. Study on local corrosion of nickel-base alloy tube in the environment of high temperature and high pressure H2S/CO2 [J]. Rare Metal Materials and Engineering, 2010, 39(3): 427-432. (in Chinese)
[7] LODDER A. Electromigration theory unified [J]. Europhysics Letters, 2005, 72(5): 774-780.
[8] Li J M, Li S L, Li J, Lin H T. Modification of solidification structure by pulse electric discharging [J]. Scripta Metallurgica et Materialia, 1994, 31(12): 1691-1694.
[9] CONRAD H. Thermally activated plastic flow of metals and ceramics with an electric filed or current [J]. Materials Science and Engineering A, 2002, 322: 100-107.
[10] LOPEZ N, CID M, PUIGGALI M. Influence of o-phase on mechanical properties and corrosion resistance of duplex stainless steels [J]. Corrosion Science, 1999, 41(8): 1615-1631.
[11] WANG Ling, DONG Jian-xin, TIAN Yu-liang, XIE Xi-shan. The investigation of macrosegregation behavior and microsegregation of alloy elements in GH3044 [J]. Rare Metal Materials and Engineering, 2006, 35(9): 1408-1411. (in Chinese)
[12] LIU Y, WANG L, WANG Y C, LIU H Y, CHEN X J, YU Y. Effects of electropulsing treatment on the precipitation behaviour of grain boundary carbides in GH3044 alloy [J]. Materials Science Forum, 2010, 654-656: 464-467.
[13] MARTA T D, PAWEL Z, ANDRZEJ P, WOJEWODA J, GUST W. Practical aspects of discontinuous precipitation and dissolution [J]. Materials Chemistry and Physics, 2003, 80(2): 476-481.
[14] ZHANG W, SUI M L, ZHOU Y Z, LI D X. Evolution of microstructures in materials induced by electropulsing [J]. Micron, 2003, 34(3-5): 189-198.
[15] ZHOU Y Z, QIN R S, XIAO S H, HE G H, ZHOU B L. Reversing effect of electropulsing on damage of 1045 steel [J]. Journal of Materials Research, 2000, 15: 1056-1061.
[16] TANG D W, ZHOU B L, CAO H, HE G H. Thermal stress relaxation behavior in thin films under transient laser-pulse heating [J]. Journal of Applied Physics, 1993, 73: 3749-3752.
[17] FENG Duan. Metal physics [M]. Book 1. Beijing: Science Press, 2000: 505. (in Chinese)
[18] ZIE?BA P, CLIFF G, LORIMER G W. Discontinuous precipitation in cobalt-tungsten alloys [J]. Acta Materialia, 1997, 45(5): 2093-2099.
[19] GEBER G P, KIRCHHEIM R. Discontinuous precipitation in a Ni-In alloy studied by analytical field ion microscopy [J]. Acta Materialia, 1997, 45(5): 2167-2175.
刘 杨1, 王 磊1, 刘红艳1, 张北江2, 赵光普2
1. 东北大学 材料各向异性与织构教育部重点实验室, 沈阳 110819;
2. 钢铁研究总院 高温材料研究所, 北京 100081
摘 要:将脉冲电流处理应用于一种镍基耐蚀合金的时效过程,研究脉冲电流对合金显微组织及耐蚀性的影响规律及机理。结果表明,脉冲电流处理后,合金在力学性能基本保持不变的前提下,其耐晶间腐蚀性能得到显著提高。脉冲电流能够显著提高合金元素的界面扩散速度,其界面扩散速度远快于体扩散速度,晶界M23C6型碳化物呈典型的不连续析出转变特征。与常规时效处理相比,脉冲电流处理后晶界碳化物的不连续析出转变,使晶界位置的合金元素的贫化程度大幅度降低,在保证合金力学性能的前提下,显著改善合金的耐晶间腐蚀能力。
关键词:镍基合金;脉冲电流;不连续析出;晶间腐蚀
(Edited by YANG Hua)
Foundation item: Project (2010CB631203) supported by National Basic Research Program of China; Project (51001021) supported by the National Natural Science Foundation of China; Projects (20100042120008, 20100042110006) supported by the PhD Programs Foundation of Ministry of Education of China
Corresponding author: WANG Lei; Tel: +86-24-83687725; E-mail: wanglei@mail.neu.edu.cn
DOI: 10.1016/S1003-6326(11)60958-8

