Low-temperature purification process of metallurgical silicon
来源期刊:中国有色金属学报(英文版)2011年第5期
论文作者:赵立新 王志 郭占成 李成义
文章页码:1185 - 1192
关键词:金属熔析法;冶金净化法;锡硅体系;太阳能级多晶硅
Key words:metal liquating method; metallurgical purification process; tin-silicon system; solar grade silicon
摘 要:硼、磷杂质的去除在冶金净化法生产太阳能级多晶硅工艺中耗能最大。金属熔析净化法可以实现冶金硅在金属液中低温下熔化,而后再结晶净化,是一种可行的低能耗硼磷去除方法。对熔析体系的选择原则进行总结,筛选出铝、锡和铟金属作为合适的熔析介质。对于Sn-Si体系,1 500 K时硼的分凝系数为0.038,远小于纯硅熔点的对应值0.8。冶金硅二次熔析净化处理可使硼的质量分数由15×10-6降至0.1×10-6,而多数金属杂质可一次性去除至0.1×10-6以下。在熔析过程中,杂质和硅生成化合物是主要的杂质去除方式。提出一种以金属熔析法为基础的低温冶金硅净化工艺。
Abstract:
The removal of B and P consumes most of heat energy in Si metallurgical purification process for solar-grade Si. Metal-liquating purification of metallurgical grade silicon (MG-Si), also called Si-recrystallization from metal liquid, was a potential energy-saving method for the removal of B and P efficiently, since Si could be melted at lower temperature by alloying with metal. The selection criteria of metal-liquating system was elaborated, and Al, Sn and In were selected out as the optimum metallic mediums. For Sn-Si system, the segregation coefficient of B decreased to 0.038 at 1 500 K, which was much less than 0.8 at the melting point of Si. The mass fraction of B was diminished from 15×10-6 to 0.1×10-6 as MG-Si was purified by twice, while that of most metallic elements could be decreased to 0.1×10-6 by purifying just once. During the metal-liquating process, the formation of compounds between impurity elements and Si was also an important route of impurity removal. Finally, one low-temperature metallurgical process based on metal-liquating method was proposed.

ZHAO Li-xin1, 2, WANG Zhi1, GUO Zhan-cheng1, 3, LI Cheng-yi4
1. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. Graduate University of Chinese Academy of Sciences, Beijing 100049, China;
3. State Key Laboratory of Advanced Metallurgy,
University of Science and Technology Beijing, Beijing 100083, China;
4. School of Chemical and Environmental Engineering,
China University of Mining and Technology, Beijing 100083, China
Received 22 October 2010; accepted 24 November 2010
Abstract: The removal of B and P consumes most of heat energy in Si metallurgical purification process for solar-grade Si. Metal-liquating purification of metallurgical grade silicon (MG-Si), also called Si-recrystallization from metal liquid, was a potential energy-saving method for the removal of B and P efficiently, since Si could be melted at lower temperature by alloying with metal. The selection criteria of metal-liquating system was elaborated, and Al, Sn and In were selected out as the optimum metallic mediums. For Sn-Si system, the segregation coefficient of B decreased to 0.038 at 1 500 K, which was much less than 0.8 at the melting point of Si. The mass fraction of B was diminished from 15×10-6 to 0.1×10-6 as MG-Si was purified by twice, while that of most metallic elements could be decreased to 0.1×10-6 by purifying just once. During the metal-liquating process, the formation of compounds between impurity elements and Si was also an important route of impurity removal. Finally, one low-temperature metallurgical process based on metal-liquating method was proposed.
Key words: metal liquating method; metallurgical purification process; tin-silicon system; solar grade silicon
1 Introduction
With the rapid development of photovoltaic industry, the main feed stock for solar cell called solar grade silicon (SoG-Si) falls into the status of serious shortage. Among new technologies for SoG-Si under researching, metallurgical process is one of the most potential appropriative methods[1]. Recently, some trial products of this process have been marketed and their solar cells have also come out. However, most of the approaches used in metallurgical process, such as slagging refining, vacuum refining and plasma treating, should be carried out at high temperature (higher than the melting point of Si) for a long time. Therefore, low-cost is still the challenge for metallurgical process and searching for new low-cost technology is always its research trend.
Metallurgical process is actually the orderly- purifying process of silicon, in which the impurities are removed step by step from metallurgical silicon (MG-Si). Because of the character difference among impurities, no method can high-effectively remove all the impurities once for all and the process is always the combination of different means[2]. The impurities inside MG-Si can be classified into two categories: some are solid-dissolved inside Si matrix and some segregate at grain boundaries. For metallic impurities, the liquid-solid segregation coefficient of which is far less than 1, most of them segregate out at Si grain boundaries. To remove the segregated impurities, acid leaching is an effective method. The removal efficiency of acid-leaching can reach up to 95%[3]. The solid-dissolved metallic impurities, especially transitional elements, though their amount is trace, can still seriously decrease the efficiency of solar cell[4]. To remove these impurities, unidirectional solidification or zone refining is an effective method. But nonmetallic impurities, especially B and P, the segregation coefficients of which are 0.8 and 0.35, respectively, are almost completely solid-dissolved inside Si matrix, so acid leaching and unidirectional solidification are not effective methods. Methods of oxidation and slag refining are perfect for the removal of B with higher content, but not competent for B with trace content[5-7]. While the plasma treatment is effective for the removal of B, the input of MG-Si must be little during purification so that B can react with reactive gas as soon as possible. Thereby the treatment capacity of plasma process is small and its energy consumption is high[8-11]. For P, the higher saturated vapor pressure by contrast with Si makes it possible to be removed by vacuum refining. However, the removal rate of P becomes very low with the decrease of its concentration. If the removal rate was accelerated by increasing the evaporation area, the loss of Si, the energy consumption and the manufacture difficulty of equipment increase rapidly[12-14]. Among the purification methods used in metallurgical process recently, Si must be molten so that impurity atoms can easily transfer within Si matrix. Long time of high-temperature operation determines its higher energy consumption, especially for the removal of B and P, and the larger segregation coefficients make them difficult to be removed by traditional segregation methods. In brief, the high-efficiency removal of B and P is actually the key of saving energy, and the low-temperature purification technology and higher removal rate of B and P are two main approaches of low-cost metallurgical process.
CHEN and PANG[15] proposed one method to remove B from MG-Si under the atmosphere of vapor/nitrogen at low temperature (900 °C). Since Si was still solid at this temperature, the transfer of impurity atoms inside Si particle was the control step during purification, and the removal efficiency of B was very low. YOSHIKAWA and MORITA[16] proposed one method of Al-Si alloying to purify MG-Si. During the purification process, MG-Si firstly melted into metal liquid and then recrystallized out. Here it is named metal-liquating process. Since Si can be melted at temperature far lower than the Si melting point, the method can make energy-saving possible. The metal- liquating method has ever been used to prepare the polycrystalline-Si thin film for solar cell, which is called liquid phase epitaxy (LPE). During LPE, poly-Si thin film grows slowly from the saturated alloying liquid of Si. Many metals such as Al, Sn and In have been used as mediums[17-19]. Commonly, the deposition rate of thin film is very slow and rigorous temperature gradient is needed so that crystals grow perfectly and few defects are developed. Like other metallurgical purification methods, metal-liquating method also could not directly purify MG-Si to meet the demand of SoG-Si, and it still needs to combine with other methods. While YOSHIKAWA and MORITA[16] adopted metal-liquating method to improve zone refining of Si, more details such as the link with other method were not stated and the proposed process was not fit for large-scale production.
In this study, the metal-liquating method using Sn-Si system including the choice of medium, the principle of purification, the separation of metal and the combination with other methods was investigated. Finally, one low-temperature metallurgical process based on metal-liquating was proposed.
2 Selection of metal-liquation system
If solid-dissolved impurities inside Si could easily transfer and could be rapidly removed under low temperature, MG-Si must be firstly melted. This can be realized just by metal-liquating method. When silicon is alloyed with metal, or say Si is dissolved into the molten metal, MG-Si could melt down at temperature even much lower than Si melting point. As the alloy liquid is cooled, Si could recrystallize and the impurities remain inside the metal liquid. The metal-liquating purification method is also the recrystallization purification of Si from metal liquid. The metal used can be regarded as the medium for metal-liquating.
The keys of the method are to melt Si under low temperature and to purify Si through recrystallization. The purpose of the method is to achieve the energy-saving purification of MG-Si under low temperature. Though many kinds of metals can dissolve Si at low temperature, only a few are the competent liquation medium. The selection criteria of media are listed below:
1) No intermediate compound generated. The generation of the intermediate compound between medium and Si would result in serious entrainment of the metal. Further, it is difficult for the latter process to separate medium, and the loss of medium would also be serious.
2) Low concentration of Si at eutectic point and low temperature of eutectic point. At eutectic point, Si would co-crystallize with metal. The eutectics are commonly binary alloy and have uniform microstructure for Si-metal systems. It is difficult to separate them and the loss of Si is serious. Furthermore, at temperature higher than the eutectic point, plenty of Si would dissolve into metal liquid. So the lower the temperature of eutectic point is, the more possible the energy-saving of purification process would be.
3) Small segregation coefficient of impurities between Si and metal. When Si recrystallizes from medium, the impurities would redistribute between Si crystal and metal liquid. Low segregation coefficient of impurities can ensure the high purification efficiency.
4) High Si-solubility of the medium at low temperature. In the liquating process, MG-Si dissolves and recrystallizes from metallic liquid, so high Si-solubility can increase the treatment capacity of the system.
5) Easy separation of Si crystal from metal liquid. Some character differences between Si and medium should be remarkable, such as the density, which makes it easy to separate Si from the wasted medium. Further, the particle size of recrystallized Si should be large and the viscidity of metal should be small, which can reduce the entrainment of medium.
Based on the above selection criteria, different metals have been investigated carefully. There are only several kinds of metals which are preferably fit for the conditions, such as Al, Sn, Zn and In. Figure 1 shows the structure of Si recrystallized from different Si-metal systems. Among these metals, YOSHIKAWA and MORITA[16] studied metal-liquating of the Al-Si and Zn-Si systems, especially Al-Si system. Since the Al-Si system has high purification efficiency and high Si-solubility, there is 50% (mass fraction) Si dissolved inside Al-Si system at 1 000 °C. The eutectic point is Si-12.5%Al (mass fraction), thereby plenty of Si would co-crystallize with Al. More importantly, Si and Al liquids have the approximate density of 2.33 and 2.31 g/mL at 950 K, respectively[20], which causes the serious loss of Si and Al and the difficulty of separation. Figure 1(a) shows that the recrystallized Si distributes uniformly inside Al. Although the research[16] proposed that the adoption of electromagnetic stirring could enrich Si, the temperature gradient was the virtual cause of enrichment. The electromagnetic stirring only accelerated the solution of MG-Si and the transfer of Si inside Al liquid.
YOSHIKAWA and MORITA[16] also studied the metal-liquating of Zn-Si system. While the recrystallized Si could depart obviously from Zn, the particle size is very small (Fig.1(b)). The vital shortcoming is the low boiling point of Zn, which is only 906 °C. During the purification process, the loss of Zn would be very serious. Further, the content of Si inside the liquid is only nearly 5% (mass fraction) at 900 °C. For In-Si system, the vital shortcoming is the high price of In.
By contrast, Sn-Si system is the optimum system. The difference of density of molten Sn and Si is remarkable, which are 7.01 and 2.33 g/mL at 505 K, respectively[20]. The melting point of Sn is 505 K, and the separation could be easily carried out at this temperature. Moreover, the recrystallized Si is large plate-like crystal and clearly gathers toward the top of system (Fig.1(c)). At 1 250 °C, the content of Si in Sn-Si reaches up to 10%[21]. The saturated vapor pressure of Sn is much lower than that of Si, so the loss of Si is little during metal-liquating. Otherwise, Sn as the impurity inside Si is electrically inactive, by contrast with other metals[17].
Figure 2 shows the microstructure of recrystallized Si from Sn-Si system before and after leaching of aqua regia. Figure 2(a) shows that the attachment amount of Sn on the surface of Si crystal is little, as the recrystallized Si is separated from molten Sn. That turns out that Si can be easily separated from molten Sn.
3 Efficiency of purification
3.1 Calculation of impurity segregation coefficient
The most important factor that determines the purification efficiency of the method is the segregation coefficient of impurity between Si and medium as Si recrystallizes from the molten metal. In this study, the coefficient was calculated via Factsage software. In the database of the software, the thermodynamic data of binary systems (Sn-Si, Sn-M and M-Si, M represents impurity element) were provided, but the data of ternary system (Sn-Si-M) were missing. During the calculation, the ternary system was extended from the relative data of binary systems. However, the main data used in its database were Cost Action 507, in which only several elements were deemed soluble in the crystalline silicon, such as B, C, Ge, N, Sn, Ti and Zn, and the others was insoluble[22]. That means that only the segregation coefficients of these elements could be calculated directly via this software. For other elements, binary data should be firstly gathered from literatures or experiments.
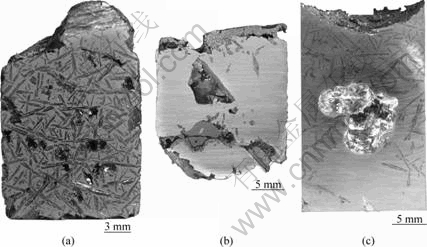
Fig.1 Optical microstructures of Si recrystallized from different alloying systems: (a) Si-50%Al; (b) Si-5%Zn; (c) Si-6%Sn
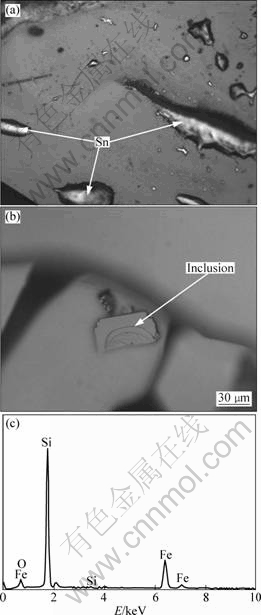
Fig.2 Microstructures of recrystallized Si from Sn-Si system before (a) and after (b) leaching by aqua regia, and EDS spectrum of inclusion (c)
Figure 3 shows the segregation coefficient of boron within Sn-Si and Al-Si systems. When Si crystallizes from alloying liquid, the segregation coefficient of B becomes much less than the initial value of 0.8. Further, the coefficient decreases with the drop of temperature. By contrast, the segregation coefficient of Sn-Si system decreases more rapidly than that of Al-Si system. Under the temperature lower than 1 500 K, the calculated segregation coefficient of Sn-Si system decreases to 0.038, which is much less than 0.8, when Si solidifies from pure melting Si at the melting point. This provides the evidence that the metal-liquating of Sn-Si system would have high efficiency for the removal of B.
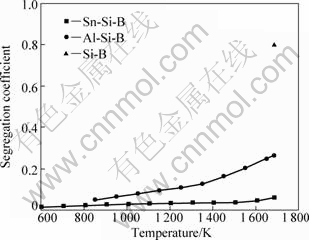
Fig.3 Segregation coefficient of boron within Sn-Si and Al-Si systems
3.2 Generation of intermediate compounds among impurities
It is known that the partition coefficient of impurity is commonly the determinant of purification efficiency during the purification process by recrystallization. Here the segregation coefficient is just the partition coefficient. However, some inclusions are discovered on the surface of the recrystallized Si in the experiments. Figures 2(b) and (c) show the microstructure of inclusions and its energy dispersive spectrum (EDS). The inclusions are attached to or embedded in Si. Since the Si crystal is leached by aqua regia, the inclusion has high acid-resistance. The EDS spectrum shows the inclusion is Fe3Si7, the intermediate compound between Fe and Si, evidencing that some other impurity-removing modes might exist during metal-liquating process.
During the solidification of pure silicon which has a constant freezing point, impurities would segregate at grain boundaries. As the solidification mode is unidirectional, the impurities would macro-segregate toward one side along the solidifying direction, which is the so-called segregation method. As Si is solidified, most of the impurities cannot form the intermediate compounds at Si freezing point, or say the compounds only possibly form at grain boundaries, where the freezing point is lower and the content of impurity is high for the effect of segregation. However, as Si is recrystallized from metallic liquid during metal-liquating process, impurities would remain in the liquid according to segregation coefficient. The good fluidity of metallic liquid is beneficial for the transfer of impurity. Because the temperature is much lower during metal-liquating, most impurities together with Si can form the intermediate compounds, which can be removed before the alloying liquid was cooled. Figure 4 shows the phase diagram of Fe-Si system. The arrow in the diagram points out that Fe can form Fe3Si7 with Si at 1 300 °C. The formation of inclusion provides evidence that the compound forms in advance, and then attaches to the surface of Si crystal as Si recrystallizes out (Fig.3(b)).

Fig.4 Phase diagram of Fe-Si system
The removal of the compounds first rapidly reduces the content of impurities in the liquid. Later, when Si recrystallizes out, the removal efficiency would further increase. This view point is similar with that proposed by YOSHIKAWA[16]. In his research, the removal mode of B was discussed. He proposed that B formed compound together with Ti, which largely boosted the removal efficiency of B. However, the mass fraction of B in MG-Si was low, commonly less than 60×10-6. In our study, no compound of TiB was detected.
3.3 Purification efficiency of metal-liquating method
1.5 g MG-Si was mixed together with 23.5 g tin (purity 99.9%) in graphite crucible. The mixture was melted under the atmosphere of argon at 1 200 °C for 1 h. Then the melt was cooled to 600 °C at a cooling rate of 3.5 °C/min. The recrystallized Si was separated out by filtration with the quartz filter. The Si crystal was leached by aqua regia to remove the attached tin, and then was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES).
Figure 5 shows the concentration of impurity of MG-Si before and after Sn-Si metal-liquating for once and twice. Commercial MG-Si was used, and its trademark was 1101. The main impurities of MG-Si and their corresponding mass fraction (10-6) were listed as: Fe 1238, Al 1054, B 15.4, Ti 195, P 223, V 155 and Mn 114.
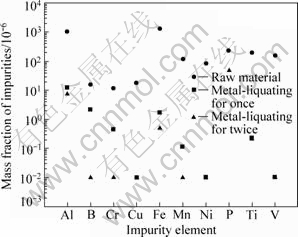
Fig.5 Concentration of impurity of MG-Si before and after Sn-Si metal-liquating
The result turns out that the metal-liquating of Sn-Si has a high removal efficiency for the metallic impurities, especially for Cu, Ni and V, up to 99.9%, with the mass fraction of less than 0.1×10-6 remained. For Fe and Al which are always the impurities with high content, the removal efficiency reached up to 99%. For Cr, Mn and Ti etc, the twice removal efficiency reached up to 99.9%.
However, for metal-liquating, it is the removal efficiency of B and P that is really concerned. The result shows that the once removal efficiency of B reaches 85%, and the removal efficiency of twice removal is more than 99.9%, and the mass fraction of B decreases from 15.4×10-6 to less than 0.1×10-6. For P, the removal efficiency of once-liquating-treatment is about 60%, and the twice removal efficiency reaches up to 85%.
The result demonstrates that it is feasible for Sn as the medium to rapidly remove B and P via metal-liquating method. In the experiment, the cooling rate is 3.5 °C/min, and almost all Si recrystallizes out at 850 °C for Sn-Si system. The time for Si recrystallization is only 2 h. Even so, the once removal efficiency of B and P reaches 85% and 60%, respectively. By contrast, the maximal operation temperature is only 1 200 °C, which is much lower than that of other methods, such as plasma treatment, slagging refining and vacuum refining, etc.
4 Problems linked to metal-liquating method
Metal-liquating method has the high removal efficiency of metallic impurities in MG-Si. But above all, the rapidly effective removal of B and P under low temperature makes the method significant. However, two important problems must be addressed, which are the solid solution of medium and the recovery of waste metal.
The solid solution of medium is the serious problem firstly. In the Si-rich side of phase diagrams, either within Al-Si system or Sn-Si system[21, 23], there are always a few Sn or Al existing inside the crystallized Si. For Al-Si system, the maximal content of Al in Si crystal is 1.2%; and for Sn-Si system, the maximal content of Sn in Si is 0.42%. Different from the metal attached to the surface, this part of Sn cannot be removed by acid leaching. So, after most impurities are removed, the newly introduced medium is a problem needed to be solved during the following process, and the difficulty level of which also determines the prospect of the method.
Former researches showed that metallic impurities can be easily removed from MG-Si, especially by segregation method. Like other metals, the solid- dissolved medium can be easily removed. For example, as the recrystallized Si is cast again, Sn would segregate at grain boundary like other metal, which can be removed by acid leaching. Above all, if B and P can be rapidly and effectively removed at lower temperature, the total energy consumption of the process would also be low.
During the metal liquating process, Si is recrystallized and separated from metal liquid. After purification, the impurities of MG-Si remain in metallic liquid. Since the impurities redistribute between medium and Si, the medium must be the high-pure metal. The usage of high pure metal must increase the cost, so the recovery of the waste metal is another problem. Compared with Si, metals commonly have lower melting point and could be easily purified. The segregation method, or say zone refining is the perfect means, especially for Sn, the melting point of which is only 231 °C. The purity of tin used in the experiments is 99.9%, which can be easily prepared in metallurgical industry. The content of B and P in Sn is the important index concerned. In our past studies, the super-gravity segregation was used to rapidly separate trace amount of impurity in metal. The segregation method can be used to recover the wasted Sn[24]. The recovery of the waste Sn can reduce the cost of metal-liquating method.
5 Low-temperature metallurgical process of MG-Si purification
Although metal-liquating method has the high purification efficiency, especially for the removal of B and P in MG-Si, the purity of Si still can not meet the demand of SoG-Si. For example, the mass fraction of most impurities is near 0.1×10-6, but still higher than 0.1×10-6 which is demanded for total content of metals in SoG-Si. Therefore, other methods must be introduced to combine with metal-liquating method, so that SoG-Si is gained finally and the total cost is reduced further.
Firstly, acid leaching should be introduced as the pretreatment in the process. MG-Si contains plenty of impurities, especially Fe and Al with mass fraction of more than 1 000×10-6, which sometimes even reaches 4 000×10-6. Acid leaching can remove almost 95% of metallic impurities, though it is ineffective for B and P. During the metal-liquating process, impurities redistribute according to the segregation coefficient. The less the content of impurity inside Sn-Si system is, the higher the purity of the purified Si is. Moreover, after purification, most of the impurities are transferred into the wasted metal. The high content of impurities would increase the recovery cost of metal. Acid leaching belongs to hydrometallurgy, and its energy consumption is low. Using acid-leaching as pretreatment to remove most of impurities can relieve the pressure of purification in the following process.
Secondly, unidirectional solidification should be introduced as the last part of the process. As Si crystallizes from metallic liquid, trace amount of metal would solid-dissolve inside Si crystal inevitably. Otherwise, the trace amount of metallic impurities still remains in recrystallized Si after the metal-liquating purification. For example, the remnants of most metals are near 0.1×10-6, while the remained mass fraction of Fe and Al can reach 5×10-6. These remained metallic impurities need to be removed further. Among the studies about metallurgical process, unidirectional solidification is regarded as the last part of the process. In our process, unidirectional solidification is still introduced as the last part of the process to remove the trace amount of remained metals.
Based on the above analysis, a low-temperature metallurgical process of MG-Si purification is proposed. Figure 6 shows the flow sheet of the low-temperature metallurgical process. The whole process can be divided into three parts: pretreatment, metal-liquating and unidirectional solidification. Within pretreatment, MG-Si is crashed and acid-leached to remove most of the metallic impurities. Within metal-liquating, the treated Si particles are melted together with Sn and then recrystallize and separate. The Si crystal is acid-leached to remove the attached Sn. The recovery of the waste Sn is also included. In this step, the content of B and P is reduced to meet the demand. Within unidirectional solidification, the recrystallized Si is purified by segregation method. The remaining metallic impurities are gathered and removed, and the purity of Si satisfies the demand of SoG-Si. In this step, the top and bottom of Si ingot can be recycled to acid-leaching of second step due to its lower content of B and P.

Fig.6 Flow sheet of low-temperature metallurgical process
6 Conclusions
1) Low energy consumption is still the challenge for metallurgical process as the special production technology of SoG-Si. Long-time removal of B and P under high temperature is the main reason. Metal-liquating purification is the potential method which can rapidly remove them under low temperature.
2) With Sn as the medium of metal-liquating method, the calculated segregation coefficient of B is less than 0.038, which is far lower than the initial value of 0.8. As Si recrystallizes from Sn-Si system at a cooling rate of 3.5 °C/min, the removal efficiencies of B, P and other metals reach more than 85%, 60% and 99.9%, respectively. During metal-liquating process, the formation of compounds between impurity elements and Si is also an important route for impurity removal. The recrystallized Si is large plate like crystal with less Sn attached, which makes it easy to separate Si from Sn liquid.
3) Two important problems relative to metal- liquating purification, the solid-solution of medium and the recovery of waste metal, were discussed. A low-temperature metallurgical process based on metal-liquating method was proposed. The process contained three parts: pretreatment, metal-liquating and unidirectional solidification.
References
[1] WODITSCH P, KOCH W. Solar grade silicon feedstock supply for PV industry [J]. Solar Energy Materials and Solar Cells, 2002, 72(1): 11-26.
[2] MORITA K, MIKI T. Thermodynamics of solar-grade-silicon refining [J]. Intermetallics, 2003, 11(11): 1111-1117.
[3] DIETL J. Hydrometallurgical purification of metallurgical-grades silicon [J]. Solar Cells, 1983, 10: 145-154.
[4] HOFSTETTER J, LELI?VRE J F, del CA?IZO C, LUQUE A. Acceptable contamination levels in solar grade silicon: From feedstock to solar cell [J]. Materials Science and Engineering B, 2009, 159-160: 299-304.
[5] WU Ji-jun, MA Wen-hui, YANG Bin, DAI Yong-nian, MORITA K. Boron removal from metallurgical grade silicon by oxidizing refining [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 463-467.
[6] KHATTAK C P, JOYCE D B, SCHMID F. A simple process to remove boron from metallurgical grade silicon [J]. Solar Energy Materials and Solar Cells, 2002, 74(1): 77-89.
[7] TANAHASHI M, NAKAHIGASHI H, TAKEDA K, YAMAUCHI C. Removal of boron from metallurgical-grade silicon by applying CaO-based flux treatment [C]// KONGOLI E, ITAGAKI K, YAMAUCHI C, SOHN H Y. Yazawa International Symposium on Metallurgical and Materials Processing: Principles and Technologies. San Diego, California: TMS, 2003: 613-624.
[8] KICHIYA S, TOMONORI K, NOBUO S. Removal of boron from metallurgical-grade silicon by applying the plasma treatment [J]. ISIJ International, 1992, 32: 630-634.
[9] BENMANSOUR M, ROUSSEAU S, MORVAN D. Effects of the DC bias in a molten silicon bath on its purification and hydrogenation by RF thermal plasma [J]. Surface and Coatings Technology, 2008, 203(7): 839-843.
[10] de WOLF S, SZLUFCIK J, DELANNOY Y, P?RICHAUD I, H??LER C, EINHAUS R. Solar cells from upgraded metallurgical grade (UMG) and plasma-purified UMG multi-crystalline silicon substrates [J]. Solar Energy Materials and Solar Cells, 2002, 72(1): 49-58.
[11] YUGE N, ABE M, HANAZAWA K, BABA H, NAKAMURA N, KATO Y, SAKAGUCHI Y, HIWASA S, ARATANI F. Purification of metallurgical-grade silicon up to solar grade [J]. Prog Photovolt: Res Appl, 2001, 9(3): 203-209.
[12] YUGE N, HANAZAWA K, NISHIKAWA K, TERASHIMA H. Removal of phosphorus, aluminum and calcium by evaporation in molten silicon [J]. The Journal of the Japan Institute of Metals, 1997, 61(10): 1086-1093.
[13] WEI Kui-xian, MA Wen-hui, DAI Yong-nian, YANG Bin, LIU Da-chun, WANG Jing-Fu. Vacuum distillation refining of metallurgical grade silicon (Ⅰ): Thermodynamics on removal of phosphorus from metallurgical grade silicon [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(s1): s1022-s1025.
[14] MA Wen-hui, WEI Kui-xian, YANG Bin, YANG Bin, LIU Da-chun, DAI Yong-nian, WANG Jing-fu. Vacuum distillation refining of metallurgical grade silicon (Ⅱ): Kinetics on removal of phosphorus from metallurgical grade silicon [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(s1): s1026-s1029.
[15] CHEN Chao, PANG Ai-suo, Removal method of boron from polycrystalline silicon. China: 101054178A [P]. 2007. (in Chinese)
[16] YOSHIKAWA T, MORITA K. Refining of silicon during its solidification from a Al-Si melt [J]. J Crystal Growth, 2009, 311: 776-779.
[17] Mc CANN M J, WEBER K J, PETRAVIC M, BLAKERS A W. Boron doping of silicon layers grown by liquid phase epitaxy [J]. Journal of Crystal Growth, 2002, 241: 45-50.
[18] KITA K, YAMATSUGU H, WEN C J, KOMIYAMA H, YAMADA K. Zone-defined growth of multicrystalline silicon film from metal-silicon solution [J]. Solar Energy Materials and Solar Cells, 2001, 65(4): 465-470.
[19] PETER K, KOPECEK R, FATH P, BUCHER E, ZAHEDI C. Thin film silicon solar cells on upgraded metallurgical silicon substrates prepared by liquid phase epitaxy [J]. Solar Energy Materials and Solar Cells, 2002, 74(2): 219-223.
[20] YASUJI K, YUTAKA S. Handbook of physico-chemical properties at high temperatures [M]. Tokyo: The Iron and Steel Institute of Japan, 1988: 2-3.
[21] JACOBS M H G, SPENCER P J. A thermodynamic evaluation of the system Si-Sn [J]. Calphad, 1996, 20(1): 89-91.
[22] BALE C W, B?LISLE E, CHARTRAND P, DECTEROV S A, ERIKSSON G, HACK K, JUNG I H, KANG Y B, MELAN?ON J, PELTON A D, ROBELIN C, PETERSEN S. FactSage thermochemical software and databases—Recent developments [J]. Calphad, 2009, 33(2): 295-311.
[23] MONDOLFO L F. Aluminum alloys structure and properties [M]. London: Butterworth, 1976: 313-315.
[24] ZHAO L X, GUO Z C, WANG Z, WANG M Y. Removal of low-content impurities from Al by super-gravity [J]. Metal Mater Trans B, 2010, 41(3): 505-508.
赵立新1, 2,王 志1,郭占成1, 3,李成义4
1. 中国科学院 过程工程研究所 湿法冶金清洁生产技术国家工程实验室,北京 100190;
2. 中国科学院 研究生院,北京 100049;
3. 北京科技大学 高效钢铁冶金国家重点实验室,北京 100083;
4. 中国矿业大学 化学与环境工程学院,北京 100083
摘 要:硼、磷杂质的去除在冶金净化法生产太阳能级多晶硅工艺中耗能最大。金属熔析净化法可以实现冶金硅在金属液中低温下熔化,而后再结晶净化,是一种可行的低能耗硼磷去除方法。对熔析体系的选择原则进行总结,筛选出铝、锡和铟金属作为合适的熔析介质。对于Sn-Si体系,1 500 K时硼的分凝系数为0.038,远小于纯硅熔点的对应值0.8。冶金硅二次熔析净化处理可使硼的质量分数由15×10-6降至0.1×10-6,而多数金属杂质可一次性去除至0.1×10-6以下。在熔析过程中,杂质和硅生成化合物是主要的杂质去除方式。提出一种以金属熔析法为基础的低温冶金硅净化工艺。
关键词:金属熔析法;冶金净化法;锡硅体系;太阳能级多晶硅
(Edited by FANG Jing-hua)
Foundation item: Project (2009BAB49B04) supported by National Key Technologies R&D Program, China
Corresponding author: GUO Zhan-cheng; Tel/Fax: +86-10-62579402, 62558489; E-mail: zcguo@metall.ustb.edu.cn
DOI: 10.1016/S1003-6326(11)60841-8

