
Preparation of ultrafine core-shell nickel spheres via self-sacrificial template
LIU Xi(刘 曦), DENG Yi-da(邓意达), SHEN Bin(沈 彬), LIU Lei(刘 磊), HU Wen-bin(胡文彬)
State Key Laboratory of Metal Matrix Composites, Shanghai Jiaotong University, Shanghai 200030, China
Received 20 December 2005; accepted 20 April 2006
Abstract: Self-sacrificial template, which is freshly precipitated Ni(OH)2 colloid, is used to fabricate ultrafine core-shell nickel spheres. The size distribution, morphology, chemical and catalyzed properties of the synthesized spheres can be controlled by regulating the preparation conditions. The varying conditions are: initial nickel ion concentration, the pH value of the solution, the scouring agent and the anion in saline solution. With very little Ni2+ concentration in the solution, the hollow spheres are formed. The higher the alkali content, the more the ultrafine colloidal cores.
Key words: ultrafine particles; core-shell; self-sacrifice; morphology
1 Introduction
Ultrafine particles stand for granules with diameter less than 0.5 ?m. For their extremely small size and large specific surface area, these sub-micron powders can be widely used as catalysts, adsorbents in the fields of chemistry, physics, biotechnology, and materials science [1-3]. Especially, those particles with specific morphologies such as core-shell spheres, hollow tubes, often exhibit novel properties which are substantially different from their solid counterparts, making them attractive from both a scientific and a technological viewpoint[4-6].
Ultrafine core-shell spheres can be fabricated via physical and chemical methods, using organic or inorganic templates and subsequently removing the templates either by calcination or decomposition in solutions[7]. For example, KIM et al[8] synthesized core-shell palladium submicrometer-sized spheres via silica sphere template which could be removed by calcinations; SUN et al[9] prepared core-shell gold spheres in HAuCl4 aqueous solutions using silver nano-particles which could be decomposed. In this investigation, we report a simple method for preparation of core-shell nickel spheres using self-sacrificial template.
In previous work, the preparation method had been reported and the forming mechanism had been discussed [10]. In this paper, more details on the regulation of the preparation method are discussed. The influence of the preparation conditions (ion as reactant, pH value of the solution, nickel ion concentration, scouring agent) on the size, morphology and chemical and catalyzed properties of the core-shell spheres is studied. The synthesized spheres are identified by field emission scanning electron microscopy(FESEM), transmission electron microscopy (TEM), X-ray diffraction(XRD), and energy dispersive X-ray microanalysis(EDX).
2 Experimental
2.1 Materials and procedure
20.00 g of nickel sulfate (99%, Shanghai Chemical Reagents Company) and 5.03 g sodium hydroxide (99%, S.C.R.C) were dissolved in 100 mL de-ionized water in beakers. The solutions were heated to 80 ℃ or so, and were mixed under violent stirring. Then a viridescent colloid was produced. 24.20 g of sodium hypophosphite (98%, S.C.R.C) was dissolved in 100 mL de-ionized water in a beaker at 80℃, and was added to the as-prepared colloid under stirring. A lot of dark-gray precipitates were produced. After the reaction, the precipitates were repeatedly washed with ammonia and de-ionized water. The final products were dried in vacuum furnace at 100 ℃ for 2 h. Then in the after- ward experiment, nickel acetate was used to replace nickel sulfate as reactant. Other reactants and the procedure were the same as the above process.
2.2 Methods of characterization
The field emission scan electron microscope images were obtained on Philips Sirion 200 (FESEM, with an acceleration voltage of 10 kV). Structural characteriza- tion was performed by means of X-ray powder diffraction (XRD, performed with Cu Kα radiation on a Rigaku D max/2000 diffractometer). Transmission electron microscopy images were recorded on a Philips CM-100 microscope (TEM, with an acceleration voltage of 100 kV). Hydrogen thermal treatment experiments were carried out on a tube furnace produced by Shanghai Experiment Electric Cooker Factory.
3 Results and discussion
3.1 Preparation conditions for ultrafine nickel particles
Traditionally, the redox reaction used in electroless nickel(EN) plating can be catalyzed by the metal Ni[11], and the deposition of nickel in plating takes couples of hours. In our study, autocatalysis of the in-process product , nickel hydroxide, was utilized to effectively cut down the time consumed in the deposition of nickel. The rapidly formed nickel precipitates are identified as micrometer and nanometer sized core-shell spheres. In the colloidal solution, Ni(OH)2 colloid has high catalytic activity similar to the metal nickel, and the minimum dissolved Ni2+ ions and 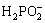 (process of equation 1) take redox reaction and generate nickel precipitates (process of equation 2) around the active core[11]. With the catalysis and sacrifice of inside Ni(OH)2 core, Ni continuously assemble outside, finally forming the Ni shell. Furthermore, the dissolution and sacrifice of nickel hydroxide depend on the degree of the catalysis, and the synthesis of core-shell spheres can be controlled by regulating the autocatalysis of the colloid core. The parameters contributing to the autocatalysis are reactant concentration, reaction temperature and pH value of the aqueous solution. The TEM image of Ni spheres in Fig.1(a) indicates the core-shell structure, with the diameter of (25±5) nm approximately. The selected diffraction pattern in the top right corner, which is of ring shape, demonstrates the amorphous structure of the powder. Fig.1(b) shows the XRD pattern of the synthesized nickel spheres. According to the EN deposition process[12], the nickel spheres are considered to be fine dispersion of microcrystalline Ni, and the shell contains very little amorphous phosphor. This is in agreement with the results reflected in the fairly broad peak of Fig.1(b).
(process of equation 1) take redox reaction and generate nickel precipitates (process of equation 2) around the active core[11]. With the catalysis and sacrifice of inside Ni(OH)2 core, Ni continuously assemble outside, finally forming the Ni shell. Furthermore, the dissolution and sacrifice of nickel hydroxide depend on the degree of the catalysis, and the synthesis of core-shell spheres can be controlled by regulating the autocatalysis of the colloid core. The parameters contributing to the autocatalysis are reactant concentration, reaction temperature and pH value of the aqueous solution. The TEM image of Ni spheres in Fig.1(a) indicates the core-shell structure, with the diameter of (25±5) nm approximately. The selected diffraction pattern in the top right corner, which is of ring shape, demonstrates the amorphous structure of the powder. Fig.1(b) shows the XRD pattern of the synthesized nickel spheres. According to the EN deposition process[12], the nickel spheres are considered to be fine dispersion of microcrystalline Ni, and the shell contains very little amorphous phosphor. This is in agreement with the results reflected in the fairly broad peak of Fig.1(b).
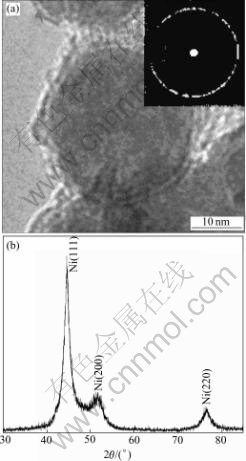
Fig.1 TEM micrograph (a) and XRD pattern (b) of core-shell Ni spheres
Ni(OH)2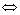 Ni2++2OH- (1)
Ni2++2OH- (1)
Ni2++ →Ni++
→Ni++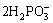 +H2 (2)
+H2 (2)
3.2 Effect of initial Ni2+ concentration
The effect of the initial nickel ion concentration on the particle is investigated. The products with initial Ni2+ concentration of 0.18 mol/L and 0.36 mol/L are studied separately. Both products are in solutions with the same concentration of 0.645 mol/L NaOH and 0.716 mol/L NaH2PO3. The totally black spheres in Fig.2(a) indicates the compact core residue. While in Fig.2(b), one sphere contains rare core residue, indicating the consumption of the core; another is hollow, indicating the exhaustion of the core. This phenomenon can be well explained by the template action of the sacrificial colloid Ni(OH)2. Since the NaOH contents are the same, the formed colloidal templates are almost equivalent, but the amounts of Ni2+ diffused to the colloid surface are different. With higher initial nickel concentration, there are more freely extricated Ni2+, and less sacrifice of the colloidal core. Correspondingly, with very little initial nickel concentration, the redox in Eqn.(2) needs more sacrifice of Ni(OH)2 to offer enough Ni2+ as reactants. Finally, the exhaustion of Ni(OH)2 core leads to the hollow shape nickel spheres.
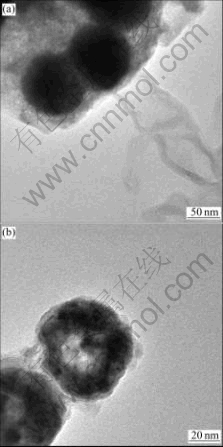
Fig.2 TEM micrographs of spheres synthesized at different concentrations of nickel sulfate: (a) 0.358 mol/L; (b) 0.179 mol/L
3.3 Effect of solution pH value
Fig.3 shows the nickel spheres with different sizes which are fabricated in solutions of different pH values. With the increase of the solution pH value, the spheres range from 600 nm (Fig.3(a)) to 30 nm (Fig.3(c)) in diameter. Fig.4 shows the direct effect of NaOH concentration on the reaction rate and temperature. With the increase of the NaOH concentration, the reaction time cuts down from 10 min to 1.5 min, and the initial reaction temperature decreases from 91 ℃ to 75 ℃.
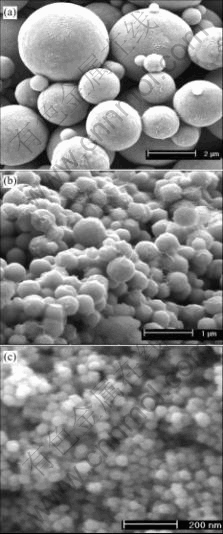
Fig.3 FESEM micrographs of spheres fabricated with different concentrations of NaOH in solutions: (a) 0.430 mol/L; (b) 0.573 mol/L; (c) 0.645 mol/L
The influence of the alkali content on the core size contributes to the changes in size of core-shell spheres. Because the alkali content is low, the reaction rate is slow, and a few nuclei are formed, which can adequately grow in to larger colloidal cores. When more hydroxyl ion can increase the reaction rate and the amount of formed nuclei, the nucleation process can complete in much short time, generating smaller colloidal cores.
During the progress of the electrolyte reaction in Eqn.(2), the pH value of the solution decreases. So add- ing more hydroxyl can get more Ni(OH)2 colloid and increase the reaction rate. With more colloid, the catalysis reactivity can be increased, and the initial activation energy of the redox will decrease. Thus the incubation period and initial temperature of the reaction decrease. This is in good agreement with the results in Fig.4.
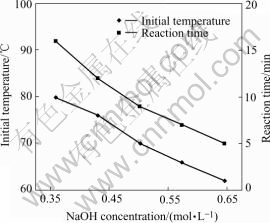
Fig.4 Effect of NaOH concentration on reaction time and temperature
3.4 Effect of scouring agent ammonia
It is well known that Ni(OH)2 deposit can be dissolved in ammonia, creating complex compound 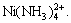 So ammonia is used as the scouring agent to remove the remained deposit. In fact, Ni(OH)2 deposit is difficult to dissolve in the ammonia electrolyte, except that there is many
So ammonia is used as the scouring agent to remove the remained deposit. In fact, Ni(OH)2 deposit is difficult to dissolve in the ammonia electrolyte, except that there is many 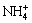 in the solution[13].
in the solution[13].  and OH- can combine to create new NH3?H2O (Eqn.(3)), promoting the complexation of nickel hydroxide and ammonia (Eqn.(4)) to react forward. The amount of
and OH- can combine to create new NH3?H2O (Eqn.(3)), promoting the complexation of nickel hydroxide and ammonia (Eqn.(4)) to react forward. The amount of 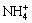 can be estimated through the pH value of ammonia. As to the electrolyte system of ammonia, when the pH value of the solution is lower than 9.25, there exists mainly
can be estimated through the pH value of ammonia. As to the electrolyte system of ammonia, when the pH value of the solution is lower than 9.25, there exists mainly 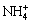 and Ni(OH)2 deposit can dissolve in the scouring agent. Correspondingly, when the pH value of the solution is higher than 9.25, there exists a little
and Ni(OH)2 deposit can dissolve in the scouring agent. Correspondingly, when the pH value of the solution is higher than 9.25, there exists a little  and Ni(OH)2 deposit is hard to dissolve in the ammonia solution. So the remained nickel hydroxide can be flocculent deposit, winding the nickel spheres. As a result, the microcosmic morphology of the nickel particle is very different when the pH value of ammonia solution is different.
and Ni(OH)2 deposit is hard to dissolve in the ammonia solution. So the remained nickel hydroxide can be flocculent deposit, winding the nickel spheres. As a result, the microcosmic morphology of the nickel particle is very different when the pH value of ammonia solution is different.
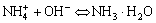 (3)
(3)
Ni(OH)2(s)+4NH3+2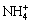 ═
═ +2H2O (4)
+2H2O (4)
Figs.5(a) and (b) show the FESEM images of nickel particles washed by ammonia solutions with different pH values. Fig.5(a) shows neat and smooth nickel spheres, and Fig.5(b) shows flocculent nickel spheres with residual Ni(OH)2 floccule.

Fig.5 FESEM micrographs of spheres washed by ammonia solutions with different pH value: (a) pH=9.05; (b) pH=11.2
3.5 Effect of different anion in saline solution
Referring to the catalytic property of the prepared nickel particle, it is noticed that the effect differs with different reactants. For example, when nickel sulfate supplies Ni2+ as reactant, the product can play the active role as catalyzer in other reactions. But when organic salt like nickel acetate supplies Ni2+ as reactant, the product becomes active only after hydrogen thermal treatment. It is probably because the nickel particles are covered by the organic matrix CH3COO-, which can decompose during the high temperate treatment in the hydrogen atmosphere[14]. The decomposition can be demonstrated from the mass loss occurring in the process of hydrogen thermal treatment and the EDX elementary analysis. During the hydrogen heating, 2.11 g as-prepared nickel powders reduce to 1.93 g after the treatment. Fig.6 shows
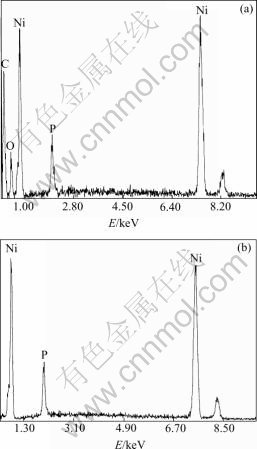
Fig.6 EDX patterns of nickel powders: (a) By heating in vacuum furnace at 100 ℃ for 2 h; (b) By hydrogen thermal treatment at 600 ℃ for 2 h
the elementary composition of nickel particles before and after the hydrogen thermal treatment. There contain carbon and oxygen elements in the as-prepared nickel powders, but there is no carbon or oxygen element after the hydrogen deoxidation.
4 Conclusions
1) Ultrafine core-shell nickel spheres are fabricated via a simple chemical method using self-sacrificial template in alkali solution environment. Nickel spheres of different size, morphologies, chemical and catalyzed properties can be obtained by regulating the preparation conditions.
2) The initial Ni2+ concentration contributes to the amount of the sacrifice of the colloidal template, thus influencing the morphology of the sphere. With very few Ni2+ in the solution, the colloidal cores can be exhausted, forming hollow shape spheres.
3) The influence of the alkali content on the core size contributes to the changes in sizes of core-shell spheres. The higher the alkali content, the faster the reduction, and the more the formed nuclei which can quickly nucleate into ultrafine colloidal cores.
4) The catalysis properties of the nickel particles covered by the organic matrix CH3COO- can act only after hydrogen thermal treatment.
References
[1] SAKIYAMA K, KOGA K, SETO T, HIRASAWA M, ORII T. Formation of size-selected Ni/NiO core-shell particles by pulsed laser ablation [J]. J Phys Chem B, 2004, 108: 523-529.
[2] HU Y, CHEN J F, CHEN W M, LI X L. Synthesis of nickel sulfide submicrometer-sized hollow spheres using a γ-irradiation route [J]. Adv Funct Mater, 2004, 14: 383-386.
[3] CORDENTE N, RESPAUD M, SENOCQ F, CASANOVE M J, AMIENS C, CHAUDRET B. synthesis and magnetic properties of nickel nanorods [J]. Nano Lett, 2001, 1: 565-568.
[4] WANG L, SASAKI T, EBINA Y, KURASHIMA K, WATANABE M. Fabrication of controllable ultrathin hollow shells by layer-by-layer assembly of exfoliated titania nanosheets on polymer templates [J]. Chem Mater, 2002, 4: 4827-4832.
[5] CHEN Z, ZHAN P, WANG Z L, ZHANG J H, ZHANG W Y, MING N B, CHAN C T, SHENG P. Two- and three-dimensional ordered structures of hollow silver spheres prepared by colloidal crystal templating [J]. Adv Mater, 2004, 6: 417-422.
[6] SUN Y, MAYERS B, XIA Y. Metal nanostructures with hollow interiors [J]. Adv Mater, 2003, 15: 641-646.
[7] ZHANG D, QI L, MA J, CHENG H. Synthesis of submicrometer- sized hollow silver spheres in mixed polymer-surfactant solutions [J]. Adv Mater, 2002, 14: 1499-1502.
[8] KIM S W, KIM M, LEE W Y, HYEON T. Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for suzuki coupling reactions [J]. J Am Chem Soc, 2002, 124: 7642-7643.
[9] SUN Y, MAYERS B, XIA Y. Template-engaged replacement reaction: a one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors [J]. Nano Lett, 2002, 2: 481-485.
[10] DENG Y D, SHEN B, LIU L, ZHAO L, LIU X, HU W B. Study on formation of submicrometer-sized hollow nickel spheres by autocatalytic reduction method [J]. Acta Chimica Sinica, 2005, 63: 1105. (in Chinese)
[11] MALLORY G O, JUAN B H. Electroless Plating: Fundamentals And Applications [M]. Orlando: American Electroplaters and Surface Finishers Society, 1990. 112-118.
[12] TAI Y L, TENG H. Template synthesis and electrochemical characterization of nickel-based tubule electrode arrays [J]. Chem Mater, 2004, 16: 338-342.
[13] YAN X S, WANG C F. Ionic Equilibrium and Chemical Reaction in Aqueous Solution [M]. Beijing: High Education Press, 1993: 75-77.(in Chinese)
[14] BOUDJAHEM A G, MONTEVERDI S, MERCY M, BETTAHAR M M. Study of support effects on the reduction of Ni2+ ions in aqueous hydrazine [J]. Langmuir, 2004, 20: 208-213.
Foundation item: Project(50474004) supported by the National Natural Science Foundation of China; Projects(0452nm046; 05nm05004) supported by the Nano Special Fund of Shanghai Science and Technology Committee, China
Corresponding author: HU Wen-bin; Tel: +86-21-62933585; E-mail: wbhu@263.net
(Edited by YANG Bing)