New insights into enhancement of sodium hypochlorite on formation and properties of anodic films on Ti6Al4V alloy
来源期刊:中南大学学报(英文版)2018年第5期
论文作者:李松梅 朱孟琪 刘建华 于美 章锦丹
文章页码:976 - 986
Key words:Ti6Al4V alloy; anodic oxidation; sodium hypochlorite; corrosion resistance; tribological properties
Abstract: Anodic films were successfully fabricated on Ti6Al4V alloy by anodic oxidation method in an environmental friendly electrolyte with and without sodium hypochlorite. The anodic films were characterized by means of the scanning electron microscope (SEM) and energy dispersive spectrometer (EDS). Results revealed that the addition of sodium hypochlorite leads to the ultrafast growth of oxide films, and results in the significant changes of morphology and thickness. The influence of sodium hypochlorite on formation and crystallization of oxide films as a function of anodizing time was discussed. Meanwhile, potentiodynamic electrochemical tests and dry sliding wear tests were performed to evaluate the corrosion resistance and tribological properties of oxide films. It was found that the oxide film fabricated with the existence of sodium hypochlorite had improved corrosion resistance and tribological properties than the one formed without sodium hypochlorite. Moreover, the effect mechanism of sodium hypochlorite on the growth rate and surface morphologies of oxide films during the anodizing process was discussed. It was found that hypochlorite ions participated in the reaction on anode which causes the rapid growth of oxide films and then affect the whole anodizing process.
Cite this article as: LI Song-mei, ZHU Meng-qi, LIU Jian-hua, YU Mei, ZHANG Jin-dan. New insights into enhancement of sodium hypochlorite on formation and properties of anodic films on Ti6Al4V alloy [J]. Journal of Central South University, 2018, 25(5): 976–986. DOI: https://doi.org/10.1007/s11771-018-3798-4.

J. Cent. South Univ. (2018) 25: 976-986
DOI: https://doi.org/10.1007/s11771-018-3798-4

LI Song-mei(李松梅), ZHU Meng-qi(朱孟琪), LIU Jian-hua(刘建华),YU Mei(于美), ZHANG Jin-dan(章锦丹)
School of Materials Science and Engineering, Beihang University, Beijing 100191, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: Anodic films were successfully fabricated on Ti6Al4V alloy by anodic oxidation method in an environmental friendly electrolyte with and without sodium hypochlorite. The anodic films were characterized by means of the scanning electron microscope (SEM) and energy dispersive spectrometer (EDS). Results revealed that the addition of sodium hypochlorite leads to the ultrafast growth of oxide films, and results in the significant changes of morphology and thickness. The influence of sodium hypochlorite on formation and crystallization of oxide films as a function of anodizing time was discussed. Meanwhile, potentiodynamic electrochemical tests and dry sliding wear tests were performed to evaluate the corrosion resistance and tribological properties of oxide films. It was found that the oxide film fabricated with the existence of sodium hypochlorite had improved corrosion resistance and tribological properties than the one formed without sodium hypochlorite. Moreover, the effect mechanism of sodium hypochlorite on the growth rate and surface morphologies of oxide films during the anodizing process was discussed. It was found that hypochlorite ions participated in the reaction on anode which causes the rapid growth of oxide films and then affect the whole anodizing process.
Key words: Ti6Al4V alloy; anodic oxidation; sodium hypochlorite; corrosion resistance; tribological properties
Cite this article as: LI Song-mei, ZHU Meng-qi, LIU Jian-hua, YU Mei, ZHANG Jin-dan. New insights into enhancement of sodium hypochlorite on formation and properties of anodic films on Ti6Al4V alloy [J]. Journal of Central South University, 2018, 25(5): 976–986. DOI: https://doi.org/10.1007/s11771-018-3798-4.
1 Introduction
Titanium and its alloys have been widely used in the aircraft, shipping and medical industry due to their high specific strength, good corrosion resistance and biocompatibility [1–4]. However, the naturally formed oxide films of titanium alloys cannot be efficient to protect themselves in some invasive situations [5]. Therefore, with simple process of technology [6] and excellent performance of oxide films [7, 8], anodic oxidation has been widely applied. Due to the main role played by the electrolyte composition in determining the characters and properties of oxide films [9–11], it has attracted increasing attention. Conventional anodic oxidation electrolytes are strong acid and alkali electrolytes such as hydroxide [5, 12], organic acid [13], sulfuric acid [14–16], phosphoric acid [17, 18]. At present, some environmentally friendly inorganic salt electrolytes have been investigated such as aluminate [19], silicate [17, 20], and chloride [18, 20]. However, compared with inorganic salt, organic salt electrolytes with better environmental performance are seldom used in anodic oxidation on titanium alloy.
In recent years, the additives in electrolytes have been found extremely influential on changing the morphologies, structures and properties of oxide films [21–24]. SO et al [21] reported that the growth rate of self-organized TiO2 nanotube layers on pure titanium was unprecedented speeded up by adding lactic acid. LALEH et al [22] found that alumina sol added into alkaline electrolyte led to less porous, more uniform morphology and enhanced corrosion resistance of oxide films. KO et al [24] found that the addition of ammonium metavanadate might cause the formation of V2O3 and V2O5 in oxide films and result in better corrosion-protection properties of samples. However, less investigation has been carried out on the effects of hypochlorite on the formation and properties of anodic films. Meanwhile, though these additives have been found promoting the growth of oxide films, the mechanism of formation during the anodizing remains unclear.
In this work, anodic films on Ti6Al4V alloy were successfully fabricated in an environmentally friendly electrolyte with and without sodium hypochlorite additives. The morphologies and phase compositions of oxide films as a function of anodizing time were studied. And the effect mechanism of sodium hypochlorite on the formation of oxide film during anodizing was discussed. Moreover, the impacts of sodium hypochlorite on film properties were studied.
2 Experimental
2.1 Materials and preparation
The elementary composition of Ti6Al4V alloy is shown in Table 1. A block of Ti6Al4V alloy was cut to provide specimens with the dimensions of 10 mm×10 mm×2 mm. Before anodic oxidation, samples were polished continuously using grit SiC papers from 150# to 2000#, cleaned ultrasonically in acetone. Then they were degreased in a mix solution of Na2SiO3 (25 g/L)+NaOH (40 g/L)+ NaCO3 (25 g/L)+Na3PO4 (40 g/L) for 10 min at 50 °C, cleaned with deionized water, and finally air dried.
Table 1 Elementary composition of Ti6Al4V alloy (wt%)

2.2 Anodization procedure
Anodic oxidation was carried out in a two- electrode electrochemical cell using a homemade DC pulse power.The titanium samples and a stainless steel were used as the working electrode and the counter electrode, respectively. The electrolytes consisted of 30 g/L sodium tartrate with or without 0.5 mL/L sodium hypochlorite (solution, 8%–10% valid chlorine content) as shown in Table 2. During anodizing, the temperature of the electrolytes was kept at 25–30 °C by a thermostatic waterbath and a magnetic stirrer. The frequency, duty ratio and the current density utilized during anodizing were 1.3 Hz, 20% and 5 A/dm2, respectively. After anodic oxidation, specimens were washed with deionized water and dried in warm air.
Table 2 Descriptions of anodizing electrolytes

2.3 Characterization of anodic oxide films
The surface and cross-section morphologies of the films were observed using a scanning electron microscope (SEM, Hitachi S-4800, Japan). The energy dispersive spectrometer (EDS) attached to the scanning electron microscope was used to determine the elementary distributions of the films.
Electrochemical tests were executed in a three-electrode cell (with anodic oxidation samples as working electrodes with an area of 1 cm2, a saturated calomel electrode as reference electrode and a platinum sheet as counter electrode) using a multi-channel potentiostat/galvanostat (Parstat 2273, USA) in a 3.5 wt% NaCl solution. The open-circuit potential (OCP) was first proceeded during immersion for 30 min; then the samples were polarized at a scan rate of 0.5 mV/s, with the scan range from 0.5 V below the OCP toward the anodic direction to a relatively high values.
The tribological test was performed with a ball-on-disc reciprocating wear tester (UMT-2, USA). The test method used a Si3N4 ceramic ball that slid in a linear, back and forth motion against a test specimen, located beneath the ball. The Si3N4 ceramic ball has a diameter of 2 mm and surface roughness about 0.01 μm. The load applied downward through the ball against the test specimen was 100 g, with a stroke length of 6 mm and frequency of oscillation of 2.5 Hz. Each sample was tested for 800 s in ambient air of normal humidity under dry conditions. After friction test, morphologies of worn surfaces were observed by SEM, and the wear of each sample was measured by electronic balance (FA2104, China). All experiments were conducted at room temperature.
3 Results and discussion
3.1 Effects of sodium hypochlorite on working potential
Figure 1 shows variations of potential with time during anodizing in sodium tartrate electrolyte with and without sodium hypochlorite. According to the potential–time curves, three regions with different slopes can be clearly distinguished. At the beginning of anodizing, the potential increases rapidly with time, which is regarded as the first stage. Because of the additive, electrical conductivity of the electrolyte increases slightly, so potential of the electrolyte containing sodium hypochlorite begins with a smaller value. It has been reported that the increasing rate of potential is proportional to the growing rate of the film [25]. As observed in Figure 1, with the addition of sodium hypochlorite, a potential curve with a bigger slope is obtained. That means that the sodium hypochlorite speeds up the growth rate of the film. After about 7 min, potential increases continually but its increasing rate slows down. In the final stage, the potential treated in the sodium tartrate electrolyte with and without sodium hypochlorite reaches 104 V and 87 V respectively and remains stable. It is clear that the thickness of the oxide film is greatly influenced by potential [26]. Therefore, the phenomenon that potential begins with a smaller value but reaches a higher one indicates the significant effects of sodium hypochlorite on promoting the formation of anodic films.
3.2 Effects of sodium hypochlorite on formation of oxide films
Figures 2 and 3 describe the SEM images of anodic oxide films on Ti6Al4V alloy formed in sodium tartrate electrolyte with and without sodium hypochlorite with different anodizing time. For samples treated in pure electrolyte, when anodizing time is 5 min, the relatively flat oxide film is obtained. And a few “flower-like” bulges are emerged (Figure 2(a)), indicating the dielectric breakdown of the oxide film [27]. Then, many more “flower-like” bulges emerge and grow larger as the anidizing time prolongs to 10 min (Figure 2(b)). As the anodizing time increases continually (30 min, Figure 2(c)), some “flower- like” bulges translate to “umbrella-like” bulges. After then, when the anodizing time increases to 60 min (Figure 2(d)), more “umbrella-like” bulges arise and their sizes become larger.
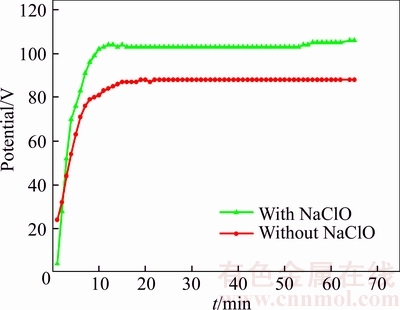
Figure 1 Variations of potential with time during anodizing in sodium tartrate electrolyte with and without sodium hypochlorite.
For samples treated in mix electrolyte, as the sample was anodized for 5 min, the similar phenomenon is observed that many big “flower-like” bulges appear(Figure 3(a)). When anodizing time increases to 10 min, surface of the oxide film is full of “flower-like” bulges which become to connect with each other(Figure 3(b)). During the whole anodizing, there is not a big diffirence of the growth rate between the “flower-like” bulges and other regions due to the addtion of sodium hypochlorite. Therefore, as anodizing time prolongs to 40 min, no relatively big “flower-like” bulges exist (Figure 3(c)) due to the connection of most bugles and the uniform growth rate of the oxide film. After then, when anodizing time inceases to 60 min, the growth rate of oxide films becomes decrease. With the sequential uniform growth, the oxide film with less microcracks is formed (Figure 3(d)) .
Cross-sectional SEM images of anodic oxide films on Ti6Al4V alloy formed in sodium tartrate electrolyte with and without sodium hypochlorite with the anodizing time of 60 min are presented in Figure 4. It can be clearly observed that the thickness values of two kinds of oxide films are relatively uniform. With the addition of sodium hypochlorite, final anodization potential of the anodized sample exhibits a 20% increase. And the oxide film with a thickness of 5 μm is obtained (Figure 4 (a)) in pure electrolyte, which is five times thinner than 25 μm of the sample anodized in mix electrolyte with sodium hypochlorite(Figure 4(b)). This is because hypochlorite ions move towards the anode during anodizing process, and react with titanium ions which makes the titanium dioxide generate quickly. Therefore, in the case of similar anodization potential values, the oxide film in mix electrolyte grows much faster, which leads to the phenomenon that potential begins with a smaller value but reaches a higher one (Figure 1) and the large difference of thickness. In a word, the cross-section SEM images and Raman spectra results imply that the addition of sodium hypochlorite leads to the ultrafast growth rate of oxide films.
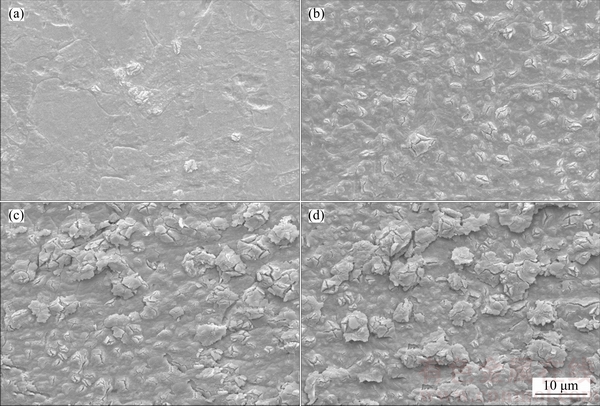
Figure 2 SEM images of anodic oxide films on Ti6Al4V alloy formed in sodium tartrate electrolyte with anodizing time of 5 min (a), 10 min (b), 30 min (c) and 60 min (d)
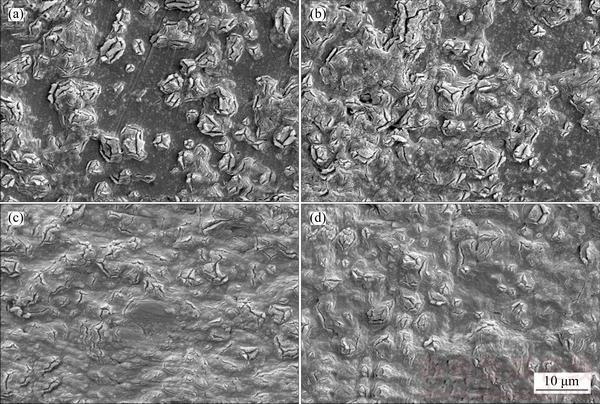
Figure 3 SEM images of anodic oxide films on Ti6Al4V alloy formed in sodium tartrate electrolyte with sodium hypochlorite with anodizing time of 5 min (a), 10 min (b), 30 min (c) and 60 min (d)

Figure 4 Cross-sectional SEM images of anodic oxide films on Ti6Al4V alloy formed in sodium tartrate electrolyte without (a) and with (b) sodium hypochlorite with anodizing time of 60 min
3.3 Possible mechanism of formation
During anodizing process, hydrolysis reactions of tartrate ions occur, and OH– ions are generated. Therefore, in the solution free from sodium hypochlorite, the following reactions normally occur on the alloy surface [28, 29]:
Ti=Ti4++4e– (anodic dissolution) (1a)
Ti4++2OH–=TiO2+2H+ (1b)
In the solution containing sodium hypochlorite, the possible reactions occurring on the alloy surface are shown below [28–30]:
Ti=Ti4++4e– (anodic dissolution) (2a)
Ti4++2OH–=TiO2+2H+ (2b)
Ti4++2ClO–+2e–=TiO2+Cl2 (2c)
Due to the weak hydrolysis of the tartaric acid ions, hydroxyl ions content is low. Therefore, during the anodizing, Eq. (1b) becomes the rate determining step of the overall reaction, and leads to the slow growth rate of the oxide film. When anodic oxidation lasts for a long time (40–60 min), growth rate of the oxide film gets a further decrease as the oxide films become thick. Therefore, the “flower-like” bulges stop growing outwards. According to other researchers, the current density at the breakdown sites is usually very high [31, 32]. Therefore, the bulge regions grow faster than the other regions. As a result, the base diameters of “flower-like” bulges increase continuously. Subsequently, the “flower-like” bulges translate to the “umbrella-like” bulges. By the contrary, as for the mix electrolytes, hypochlorite ions move towards the anode during anodizing process. Then Eq. (2c) generated, and the overall reaction speed increases significantly. This is because the hypochlorite ions content is much higher than hydroxyl ions content. Therefore, the growth rate of oxide films is greatly enhanced. As the anodizing time prolongs, the “flower-like” bulges grow bigger rapidly and connect with each other. Then thick oxide films were obtained and the formation of Cl2 gas should be one reason of the surface cracks.
Figure 5 presents the element composition of oxide films formed in the sodium tartrate electrolyte with sodium hypochlorite with different anodizing time. It is clearly observed that peak Cl presents with different anodizing time. And the content percentages of Cl are basically same, which supports the generation of Eq. (2c).
3.4 Corrosion resistance of anodized films
The polarization curves of oxide films formed in sodium tartrate electrolyte with or without sodium hypochlorite in 3.5 wt% NaCl solution are shown in Figure 6. The corrosion protection (φcorr) and passive current density (Jcorr) values given in Table 3 are extracted directly from the polarization curves by extrapolation. By combining Figure 6 and Table 3, it can be obviously confirmed that the corrosion resistance of the sample treated in the mix electrolyte is much enhanced compared with the sample treated in pure electrolyte. For the sample treated in mix electrolyte, the corrosion protection has a significant shift to positive direction (134 mV) and the passive current density is reduced more than three times.
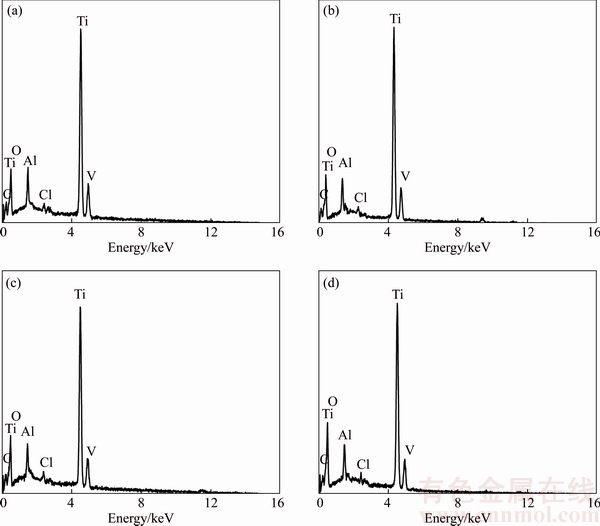
Figure 5 EDS of surface of anodic oxide films on Ti6Al4V alloy formed in sodium tartrate electrolyte with sodium hypochlorite with anodizing time of 5 min (a), 10 min (b), 30 min (c) and 60 min (d)
Figure 7 demonstrates the impedance modulus and phase angle of the oxide films formed in sodium tartrate electrolyte with or without sodium hypochlorite in 3.5 wt% NaCl solution. As shown in Figure 7(a), the impedance of the oxide film fabricated in mix electrolyte is obviously smaller than that of the oxide film formed in pure electrolyte. Furthermore, a shift to higher frequencies is evident in Figure 7(b) which can relate to the change of surface brought by sodium hypochlorite. These results indicated that the corrosion resistance of the sample fabricated in mix electrolyte is much better.
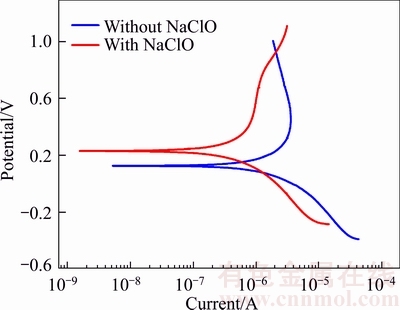
Figure 6 Polarization curves of anodic oxide films formed in sodium tartrate electrolyte without and with sodium hypochlorite.
Table 3 Electrochemical data of samples with different oxide films from potentiodynamic polarization tests
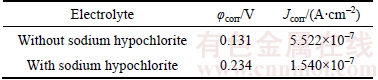
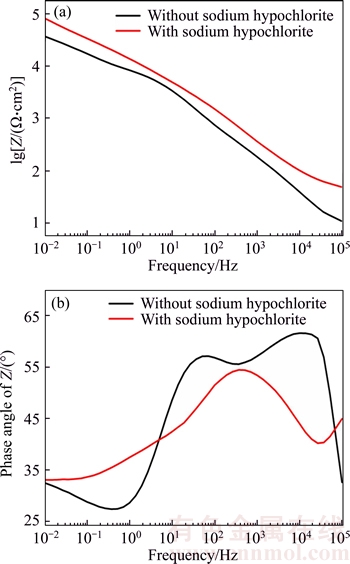
Figure 7 Impedance modulus (a) and phase angle (b) of oxide films formed in sodium tartrate electrolyte with and without sodium hypochlorite in 3.5 wt% NaCl solution
The improved performance offered by the sample treated in mix electrolyte is attributed to the big thickness and less cracks of the oxide film, which avoid the surface cracks from directly going deep into the substrate and provides a more compact structure which acts as a barrier that blocks access to the film.
Figure 8 presents the crack morphologies of samples treated in sodium tartrate electrolyte without and with sodium hypochlorite before and after potentiodynamic polarization tests. It is clearly shown in cracks that the structure of oxide films translates from layered structure (Figures 8(a) and (c)) to honeycomb structure (Figures 8(b) and (d)) before and after potentiodynamic polarization tests, indicating the corrosion occurred in cracks. Compared with the sample treated in pure electrolyte (Figure 8(b)), honeycomb area of the sample treated in mix electrolyte (Figure 8(d)) is obviously small. This further indicates the enhanced corrosion resistance of the sample treated in mix electrolyte.
3.5 Tribological properties
The friction coefficient curves of the samples untreated and anodized in sodium tartrate electrolyte without and with sodium hypochlorite under dry condition are shown in Figure 9. It is obviously presented that the sample anodized in pure electrolyte has a very similar friction coefficient curve to that of the untreated sample, indicating that the oxide film is worn through in a short time due to the small thickness. For the oxide film anodized in mix electrolyte, a much lower friction coefficient is obtained. There may be two reasons. Firstly, the oxide film fractures and deforms during wear process, then large number of detrital-matters generate and lubricate between the contact areas. Secondly, detrital-matters deform and bond together under the action of the force, and a relatively compact lubricating layer is formed gradually.
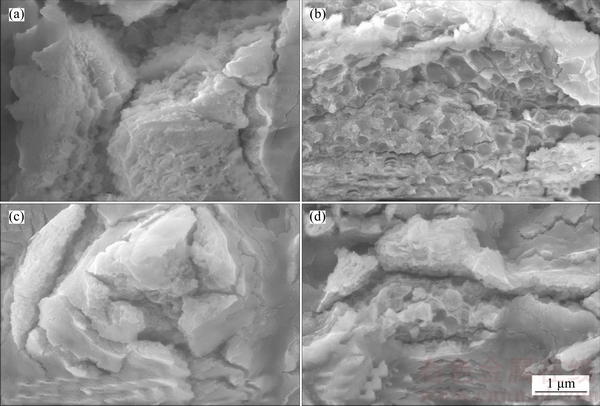
Figure 8 Morphologies of cracks of samples treated in sodium tartrate electrolyte without sodium hypochlorite before (a) and after (b) potentiodynamic polarization tests, and with sodium hypochlorite before (c) and after (d) potentiodynamic polarization tests
Figure 10 demonstrates the worn surfaces of the samples untreated and anodized in sodium tartrate electrolyte without and with sodium hypochlorite. As shown in Figure 10, the width of worn surface of the sample anodized in pure electrolyte is similar to that of the untreated sample. And the worn surfaces of samples untreated and anodized in pure electrolyte are bright and full of scratches, which supports the conclusion that the oxide film was worn through in a short time. By contrary, the sample treated in mix electrolyte has a much narrower worn surface without apparent scratches. And the enlarged drawing of worn surface of the sample shown in Figure 10(d) implies the formation of lubricating layer.
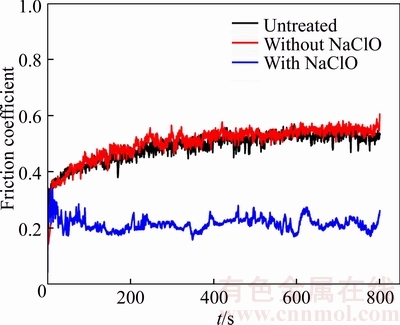
Figure 9 Friction coefficient curves of samples untreated and anodized in sodium tartrate electrolyte without and with sodium hypochlorite under dry condition
Wear rates of the samples untreated and anodized in sodium tartrate electrolyte without and with sodium hypochlorite are shown in Figure 11. It can be clearly observed that the sample anodized in pure electrolyte has a second largest wear rate which is just a little smaller than that of the untreated sample. The oxide film fabricated in mix electrolyte has a much smaller wear rate than the other two kinds of samples. Indicating that the oxide film formed in mix electrolyte is efficient to protect samples from wearing. The tribological test results prove that the addition of sodium hypochlorite into electrolyte leads to much improved tribological properties of samples.
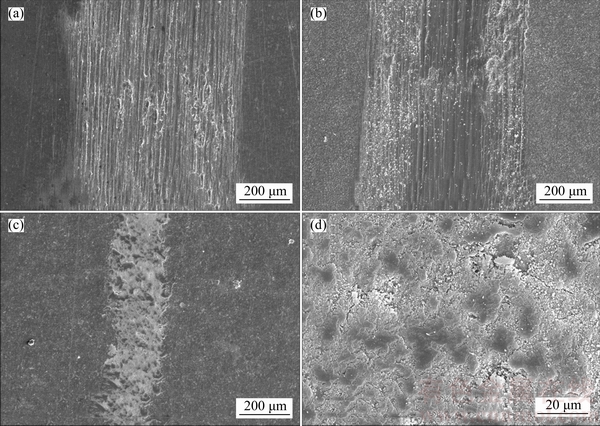
Figure 10 Worn surfaces of samples untreated (a) and anodized in sodium tartrate electrolyte without (b) and (c, d) with sodium hypochlorite
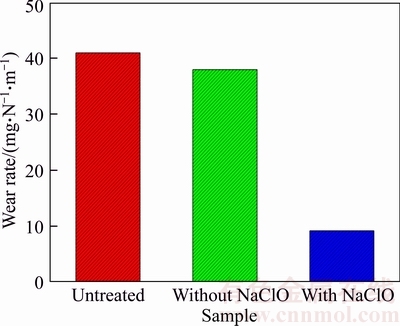
Figure 11 Wear rates of samples untreated and anodized in sodium tartrate electrolyte without and with sodium hypochlorite.
4 Conclusions
Oxide films with different morphologies were fabricated on Ti6Al4V alloy in sodium tartrate electrolyte with and without sodium hypochlorite. And the surface morphologies, structures and phase compositions of oxide films fabricated in different electrolytes with different anodizing time were studied, respectively. And much more improved growth rate of oxide films was obtained when sodium hypochlorite was added into base electrolyte. The promoting mechanism of sodium hypochlorite on the formation of oxide films was discussed that hypochlorite ions participated in the reaction on anode and leaded to great increase of overall reaction during anodizing. Then the rapid growth of oxide film was obtained. Electrochemical measurement and tribological test indicated that the oxide film formed in sodium tartrate electrolyte with sodium hypochlorite had much enhanced corrosion resistance and tribological properties.
References
[1] HUANG Ping, WANG Fei, XU Ke-wei, HAN Yong. Mechanical properties of titania prepared by plasma electrolytic oxidation at different voltages [J]. Surface & Coatings Technology, 2007, 201: 5168–5171.
[2] LIU Jue, LI Jing, YANG Hai-lin, RUAN Jian-ming. Microstructure of titanium-nickel alloy by mechanical alloying [J]. Journal of Central South University: Science and Technology, 2015, 46(4): 1201–1207. (in Chinese)
[3] SHARIFI H, ALIOFKHAZRAEI M, GHASEN B D, SABOUR ROUHAGHDAM A. Characterization of PEO nanocomposite coatings on titanium formed inelectrolyte containing atenolol [J]. Surface & Coatings Technology, 2016, 304: 438–449.
[4] LIU Jian-hua, WU Liang, YU Mei, LI Song-mei, WU Guo-long. Effects of sealing process on corrosion resistance and roughness of anodic films of titanium alloy Ti-10V-2Fe-3A1 [J]. Journal of Central South University, 2011, 18(6): 1795–1801.
[5] CHEN Jia-li, WANG Jin-wei, YUAN Hong-ye. Morphology and performances of the anodic oxide films on Ti6Al4V alloy formed in alkaline-silicate electrolyte with aminopropyl silane addition under low potential [J]. Applied Surface Science, 2013, 284: 900–906.
[6] ALADIEM A. Anodic oxidation of titanium and its alloys [J]. Journal of Material Science, 1997, 8: 688–704.
[7] LI Song-mei, LIU Jian-hua, YU Mei, WU Liang, YANG Kang. Microstructure and abrasive wear behaviour of anodizing composite films containing SiC nanoparticles on Ti6Al4V alloy [J]. Journal of Central South University, 2014, 12: 4415–4423.
[8] WANG Yan, WU Yu-cheng, QIN Yong-qiang, XU Gao-bin, HU Xiao-ye, CUI Jie-wu, ZHENG Hong-mei, HONG Yu, ZHANG Xin-yi. Rapid anodic oxidation of highly ordered TiO2 nanotube arrays [J]. Journal of Alloys and Compounds, 2011, 509: L157–L160.
[9] ZHANG R F, SHI H W, LIU Z L, ZHANG S F, ZHANG Y Q, GUO S B. Property of anodic coatings obtained in an organic, environmental friendly electrolyte on aluminum alloy 2024-T3 [J]. Applied Surface Science, 2014, 289: 326–331.
[10] FORNO A D, BESTETTI M. Effect of the electrolytic solution composition on the performance of micro-arc anodic oxidation films formed on AM60B magnesium alloy [J]. Surface & Coatings Technology, 2010, 205: 1783–1788.
[11] SIMKA W, SOWA M, SOCHA R P, MACIEJA A, MICHALAKA J. Anodic oxidation of zirconium in silicate solutions [J]. Electrochimica Acta, 2013, 104: 518–525.
[12] BAGHERI H R, ALIOFKHAZRAEI M, GHEYTANI M, MASIHA H R, SABOUR ROUHAGHDAM A, SHAHRABI T. Growth and internal microstructure of micro-arc oxidized MgO-based nanocomposite [J]. Coating Surface & Coatings Technology, 2015, 283: 1–9.
[13] REN Jian-jun, ZUO Yu. The anodizing behavior of aluminum in malonic acid solution and morphology of the anodic films [J]. Applied Surface Science, 2012, 261: 193–200.
[14] OHTSUKA T, MASUDA M, SATO N. Ellipsometric study of anodic oxide films on titanium in hydrochloric acid, sulfuric acid, and phosphate solution [J]. Journal of the Electrochemical Society, 1985, 132: 787–792.
[15] GARSIVAZ JAZI M R, GOLOZAR M A, RAEISSI K, FAZEL M. Evaluation of corrosion and tribocorrosion of plasma electrolytic oxidation treated Ti–6Al–4V alloy [J]. Surface & Coatings Technology, 2014, 244: 29–36.
[16] YETIM A F. Investigation of wear behavior of titanium oxide films, produced by anodic oxidation, on commercially pure titanium in vacuum conditions [J]. Surface & Coatings Technology, 2010, 205: 1757–1763.
[17] POLAT A, MAKARACI M, USTA M. Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate [J]. Journal of Alloys and Compounds, 2010, 504: 519–526.
[18] GHEYTANI M, ALIOFKHAZRAEI M, BAGHERI H R, MASIHA H R, ROUHAGHDAM A S. Wettability and corrosion of alumina embedded nanocomposite MAO coating on nanocrystalline AZ31B magnesium alloy [J]. Journal of Alloys and Compounds, 2015, 649: 666–673.
[19] WANG Zhi-jiang, WU Li-na, CAI Wei, JIANG Zhao-hua. Effects of fluoride on the structure and properties of microarc oxidation coating on aluminium alloy [J]. Journal of Alloys and Compounds, 2010, 505: 188–193.
[20] KHORASANIAN M, DEHGHAN A, SHARIAT M H, BAHROLOLOOM M E, JAVADPOUR S. Microstructure and wear resistance of oxide coatings on Ti–6Al–4V produced by plasma electrolytic oxidation in an inexpensive electrolyte [J]. Surface & Coatings Technology, 2011, 206: 1495–1502.
[21] SO S, LEE K, SCHMUKI P. Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes [J]. Journal of the Electrochemical Society, 2012, 134: 11316- 11318.
[22] LALEH M, SABOUR ROUHAGHDAM A, SHAHRABI T, SHANGHI A. Effect of alumina sol addition to micro-arc oxidation electrolyte on the properties of MAO coatings formed on magnesium alloy AZ91D [J]. Journal of Alloys and Compounds, 2010, 496: 548–552.
[23] SREEKANTH D, RAMESHBABU N, VENKATESWARLU K, SUBRAHMANYAM C, RAMA KRISHNA L, PRASAD RAO K. Effect of K2TiF6 and Na2B4O7 as electrolyte additives on pore morphology and corrosion properties of plasma electrolytic oxidation coatings on ZM21 magnesium alloy [J]. Surface & Coatings Technology, 2013, 222: 31–37.
[24] KO Y, LEE K, SHIN D. Effect of ammonium metavanadate on surface characteristics of oxide layer formed on Mg alloy via plasma electrolytic oxidation [J]. Surface & Coatings Technology, 2013, 236: 70–74.
[25] BAI Allen, CHEN Zhi-jia. Effect of electrolyte additives on anti-corrosion ability of micro-arc oxide coatings formed on magnesium alloy AZ91D [J]. Surface & Coatings Technology, 2009, 203: 1956.
[26] ZHANG S F, ZHANG R F, LI W K, LI M S, YANG G L. Effects of tannic acid on properties of anodic coatings obtained by micro arc oxidation on AZ91 magnesium alloy [J]. Surface & Coatings Technology, 2012, 207: 170–176.
[27] YAHALOM J, ZAHAVI J. Electrolytic breakdown crystallization of anodic oxide films on Al, Ta and Ti [J]. Electrochimica Acta, 1970, 15: 1429–1435.
[28] HWANG B J, HWANG J R. Kinetic model of anodic oxidation of titanium in sulphuric acid [J]. Journal of Applied Electrochemistry, 1993, 23: 1056–1062.
[29] DURDU S, DENIZ  F, KUTBAY I, USTA M. Characterization and formation of hydroxyapatite on Ti6Al4V coated by plasma electrolytic oxidation [J]. Journal of Alloys and Compounds, 2013, 551: 422–429.
F, KUTBAY I, USTA M. Characterization and formation of hydroxyapatite on Ti6Al4V coated by plasma electrolytic oxidation [J]. Journal of Alloys and Compounds, 2013, 551: 422–429.
[30] HASSAN F M B, NANJO H, VENKATACHALAM S, KANAKUBO M, EBINA T. Effect of the solvent on growth of titania nanotubes prepared by anodization of Ti in HCl [J]. Electrochimica Acta, 2010, 55: 3130–3137.
[31] XING Jun-heng, XIA Zheng-bin, HU Jian-feng, ZHANG Yan-hong, ZHONG Li. Growth and crystallization of titanium oxide films at different anodization modes [J]. Journal of the Electrochemical Society, 2013, 160: C239–C246.
[32] XING Jun-heng, XIA Zheng-bin, HU Jian-feng, ZHANG Yan-hong, ZHONG Li. Time dependence of growth and crystallization of anodic titanium oxide films in potentiostatic mode [J]. Corrosion Science, 2013, 75: 212–219.
(Edited by YANG Hua)
中文导读
次氯酸钠对Ti6Al4V合金表面阳极氧化膜的形成和性能的影响
摘要:在环保型的槽液中有无添加次氯酸钠的情况下都成功制备了Ti6Al4V合金表面的阳极氧化膜。使用扫描电镜、能谱仪对制备的阳极氧化膜进行表征。结果显示,添加次氯酸钠可以引起膜层的快速生长以及膜层形貌厚度的显著变化。研究了随着时间的变化次氯酸钠对膜层的形成和结晶度的影响。同时,采用动电位极化和干摩擦滑动测试来评估阳极氧化膜的耐蚀和耐磨性能。结果发现,在次氯酸钠存在的条件下形成的膜层具有较好的耐蚀和耐磨性能。对次氯酸钠作用于氧化膜的生长速率和表面形貌的影响机制也进行了研究,发现在阳极氧化过程中,次氯酸根离子参与了阳极的反应,因此引起了氧化膜的快速生长进而影响整个阳极氧化过程。
关键词:Ti6Al4V合金;阳极氧化;次氯酸钠;耐蚀性;摩擦性能
Foundation item: Project(51271012) supported by the National Natural Science Foundation of China
Received date: 2016-09-29; Accepted date: 2017-02-23
Corresponding author: LI Song-mei, PhD, Professor; Tel/Fax: +86–10–82317103; E-mail: songmei_li@buaa.edu.cn; ORCID: 0000- 0002-3729-9398

