文章编号:1004-0609(2014)10-2634-08
铁酸锌选择性还原的反应机理
侯栋科1,彭 兵1, 2,柴立元1, 2,彭 宁1,闫 缓1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083)
摘 要:通过TG、物相分析、XPS、XRD、SEM-EDS等手段研究铁酸锌选择性还原反应机理,考察铁酸锌质量损失和分解特征、物相转变过程和产物层形貌变化以及Zn2+与Fe2+的离子迁移行为。结果表明:反应表现为失氧过程,还原产生的Fe2+使铁酸锌分解产生ZnO,ZnO含量与Fe2+含量线性相关。Fe2+向铁酸锌内部迁移替代Zn2+,Zn2+则向外部迁移并富集于表面,促使ZnO在表面形成。铁酸锌逐步向磁铁矿转变,Fe2+的嵌入和锌的迁出使铁酸锌晶胞参数先增大后减小,还原产物为ZnO和含锌的磁铁矿。颗粒产物层中的还原产物相互夹杂,并包裹着未反应的铁酸锌。
关键词:铁酸锌;选择性还原;相变过程;离子迁移
中图分类号:TF813 文献标志码:A
Selective reduction mechanism of zinc ferrite
HOU Dong-ke1, PENG Bing1, 2, CHAI Li-yuan1, 2, PENG Ning1, YAN Huan1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China)
Abstract: The selective reduction mechanism of zinc ferrite was studied by TG, phase analysis, XPS, XRD and SEM-EDS. The mass loss, decomposition features of zinc ferrite, ionic migration behavior of Zn2+ and Fe2+ ions, phase transformation process and morphological change of product layer were investigated. The results show that reaction is oxygen loss process. The reduced Fe2+ made ZnO separated from zinc ferrite, the content of ZnO has a significant linear correlation with the content of Fe2+. Fe2+ migrates into the inner zinc ferrite to substitute Zn2+, while Zn2+ moves out and enriches on the surface, promoting the formation of ZnO. Zinc ferrite transforms to magnetite, the lattice parameter of zinc ferrite increases and then decreases due to the intercalation of Fe2+ and the emigration of Zn2+. The reduction products are zinc oxide and zinc-containing magnetite. The reduction products coexist with each other in the product layer and unreacted zinc ferrite is covered with the product layer.
Key words: zinc ferrite; selective reduction; phase transformation; ionic migration
铁酸锌是传统湿法炼锌浸出渣和电弧炉炼钢粉尘中的主要成分,资源化利用含铁酸锌物料的关键在于处理其中的铁酸锌。选择性还原焙烧是处理含铁酸锌废渣的一种新方法,该方法是将渣中的铁酸锌选择性还原分解为ZnO和磁铁矿,然后通过中性浸出或磁选工艺实现铁锌分离[1-2]。
铁酸锌的选择性还原焙烧在中温(600~800 ℃)和弱还原性气氛条件下进行[3],与传统的回转窑烟化法相比,该方法具有还原温度低、可同步回收锌铁的优点。为了深化对选择性还原焙烧工艺的认识,需研究铁酸锌的选择性还原反应机理。
铁酸锌的还原反应涉及Fe-Zn-O系的物相转变[4],主要物相有氧化铁(Fe2O3)、铁酸锌(ZnFe2O4)、磁铁矿(Fe3O4)、方铁矿(Fe1-xO)、单质铁、氧化锌和单质锌。铁酸锌的还原不同于氧化铁的还原,在还原过程中不存在氧化铁的物相[5]。铁酸锌和磁铁矿均为尖晶石结构,可形成连续的固溶体。方铁矿是缺陷的NaCl型晶体[6],其内部分布有填隙铁离子构成的缺陷簇,当x=0时,为晶型完整的FeO。缺陷结构的方铁矿可固溶部分锌形成Fe1-xZnxO,不利于后续的铁锌分离,因而,在工艺中需避免磁铁矿过还原形成方铁矿[7]。单质锌和铁是高温和强还原性条件下的产物,在选择性还原条件下不予考虑。
虽然铁酸锌和磁铁矿结构相同,但其中的离子分布不同。由于Zn2+和Fe2+分别倾向于占据尖晶石结构中的四面体和八面体间隙[8],因此,铁酸锌中Zn2+全部位于四面体间隙,而磁铁矿中的Fe2+则替代一半的Fe3+占据八面体间隙,尖晶石结构中的这两种离子占位分别称为正尖晶石和反尖晶石。离子种类和含量的不同使铁酸锌和磁铁矿的晶胞参数有着明显差异,可以据此判断还原过程中尖晶石的结构转变。铁酸锌在低温(300 ℃)下的经H2还原可生成氧缺位铁酸锌(ZnFe2O4-δ),ZnFe2O4-δ在CO2分解沉积碳[9-10]和H2S脱硫的废气治理工艺[11]中有着广泛应用,ZnFe2O4-δ中由于还原生成的Fe2+在尖晶石晶格当中的占位会使晶胞参数增大[12]。非化学计量比的铁酸锌中锌含量的不同也会造成晶胞参数的变化[13]。
铁酸锌的还原过程中存在着铁、锌离子的迁移,TONG[14]认为铁酸锌还原产生的Fe2+由于化学势梯度的作用会由表面向内部迁移,同时,Zn2+从内部向表面的扩散并以锌蒸气的形式从尖晶石中分离,但其离子扩散的结论缺乏直接的实验依据。离子的扩散和迁移在固相反应中普遍存在,采用XPS研究发现,铁酸锌的固相合成反应中Zn2+由ZnO向Fe2O3的晶格中迁移,并同时占据晶格中四面体和八面体间隙,使XPS峰的峰型宽化,经过长时间的退火过程才能消除这一原子占位混乱[15],机械活化同样也存在占位混乱的晶格畸变现象[16-18]。
本文作者在上述研究的基础上,开展了铁酸锌选择性还原的反应机理研究。通过热力学分析确定铁酸锌选择性还原的优势区域,采用热重和化学分析研究失氧和分解特征,借鉴XPS表面分析的研究手段对离子迁移行为进行探究,利用XRD及晶胞参数的分析查明物相转变过程,并检测产物层的形貌变化以进一步说明其还原机理。
1 实验
1.1 铁酸锌合成方法
将分析纯的ZnO和Fe2O3按摩尔比1:1充分混匀,在1000 ℃下煅烧4 h后,用1 mol/L的盐酸在40 ℃下浸出1 h,以除去未反应的氧化物,然后在1000 ℃下煅烧1 h,退火均采用自然随炉冷却。发生的主要反应为ZnO+Fe2O3→ZnFe2O4,合成样品细磨筛分至粒径小于74 μm,干燥1 h后用于还原焙烧实验。图1所示为合成铁酸锌的XRD谱。由图1可见,衍射峰峰型尖锐,晶型完好。
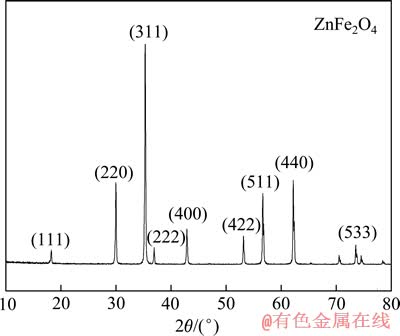
图1 合成铁酸锌的XRD谱
Fig. 1 XRD pattern of synthetic zinc ferrite
1.2 还原焙烧实验
还原焙烧在Netzsch (STA449F3)热重分析仪中进行,称取3 g铁酸锌,在惰性氩气流中以10 ℃/min的加热速率升温至预定温度。然后按设定的CO浓度(为CO占CO+CO2+Ar总体积的体积分数(φ1(CO))以及CO占CO+CO2总体积的体积分数(φ2(CO))向气流中添加CO和CO2,并调节氩气流量以构成100 mL/min的气流。反应一段时间后,停止通入CO和CO2,样品在氩气流中冷却至室温。分析不同还原时间还原产物中的ZnO和Fe2+,分别用EDTA和重铬酸钾滴定法进行分析[19],并通过热重检测还原过程中的质量损失特征。
1.3 检测表征方法
还原产物用X射线光电子能谱分析其表面性质,检测使用ESCALAB 250Xi系统(Thermo Fisher Scientific)。采用Al Kα X射线源,通过能量为20 eV,结合能以C1s峰的284.6 eV为标准进行校正。数据分析采用Thermo Avantage Software软件,获得了不同还原时间下还原产物的Zn 2p3/2、Fe 2p的XPS谱和表面的摩尔分数。
还原产物用X射线衍射仪(Rigaku, TTR-Ⅲ)进行物相分析,衍射仪采用Cu Kα辐射源(λ=1.5406  ),扫描角度10°~80°,步长0.02 (°)/step,扫描速度10 (°)/min。高角度衍射峰通常更能反映晶格结构的变化,从图1可见,(440)衍射峰在高角度区强度最高,可用于分析衍射峰的变化。同时,采用科恩最小二乘法[20-21]计算晶胞参数,用Matlab7.0编程进行计算。
),扫描角度10°~80°,步长0.02 (°)/step,扫描速度10 (°)/min。高角度衍射峰通常更能反映晶格结构的变化,从图1可见,(440)衍射峰在高角度区强度最高,可用于分析衍射峰的变化。同时,采用科恩最小二乘法[20-21]计算晶胞参数,用Matlab7.0编程进行计算。
还原产物用JSM-6360LV型扫描电子显微镜及GENESIS 60S型能谱仪对产物颗粒层形貌及元素构成进行分析,还原产物用树脂胶结,磨平抛光后进行电镜观测及能谱分析。
2 结果与讨论
2.1 热力学优势区域图分析
铁酸锌与CO还原过程中可能发生的主要反应如下:
3ZnFe2O4+CO→3ZnO+2Fe3O4+CO2 (1)
Fe3O4+CO→3FeO+CO2 (2)
FeO+CO→Fe+CO2 (3)
ZnO+CO→Zn+CO2 (4)
铁酸锌的选择性还原反应可用反应(1)表示,要实现选择性需在反应(1)进行的同时避免反应(2)~(4)的发生,以防止磁铁矿和ZnO的过还原导致的后续铁锌分离困难。图2所示为热力学计算软件Factsage所绘制的Zn-Fe-C-O系在750 ℃下的优势区域图,可见在750 ℃下能发生反应(1)~(3),反应(4)则得到有效抑制,铁酸锌的还原是ZnFe2O4→Fe3O4+ZnO→FeO+ ZnO→Fe+ZnO的连续物相转变。图中反应(1)和(2)之间的区域为Fe3O4+ZnO的优势区域,反应(1)和(2)的lg[p(CO)]与lg[p (CO2)]的关系式分别为
Lg[p(CO)]=lg[p(CO2)]-1.5593 (5)
Lg[p(CO)]=lg[p(CO2)]-0.246434 (6)
计算可得反应(1)和(2)在平衡条件下的气相组成,φ2(CO)分别为2.68%和36.18%。为了实现选择性还原,在750 ℃下应使反应气氛条件在Fe3O4+ZnO的优势区域内,即控制φ2(CO)在2.68%~36.18 %之间。图中★代表的反应条件(φ1(CO)=8%和φ2(CO)=20%)在Fe3O4+ZnO的优势区域内,因而,选择该条件为还原机理研究的反应气氛条件。
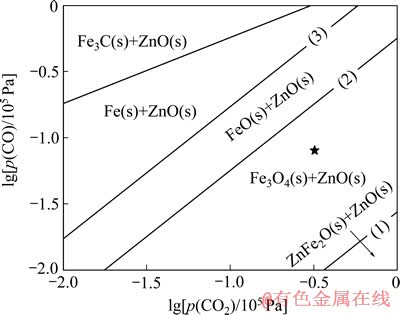
图2 750 ℃下Zn-Fe-C-O系的优势区域图
Fig. 2 Predominance-area diagram of Zn-Fe-C-O at 750 ℃
2.2 质量损失及分解特征
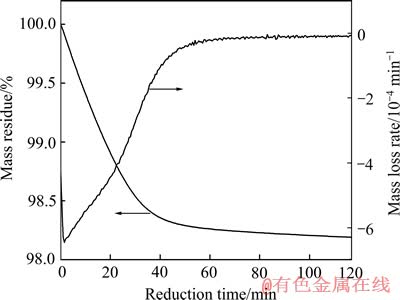
图3 750 ℃下铁酸锌选择性还原的等温质量损失和微分质量损失曲线
Fig. 3 Isothermal TG-DTG curves of selective reduction of zinc ferrite at 750 ℃
还原反应表现为质量损失过程,质量损失主要是因为铁酸锌的还原失氧。图3所示为750 ℃下铁酸锌选择性还原的等温质量损失和微分质量损失曲线。由图3可见,0~40 min铁酸锌持续快速质量损失,此时质量损失率为1.65%,之后曲线平缓下降,120 min后最终质量损失率为1.82%,低于反应(1)的理论质量损失率为2.21%,说明反应尚未进行完全。由DTG曲线可见,反应1.5 min内,质量损失速率快速增大至-0.64%/min,这是由于CO在颗粒层中的扩散限制了最初期的反应速率,其后反应速率开始持续降低;60 min后,质量损失速率减小为-2×10-5 min-1,反应近乎停滞。反应最终达到平衡的原因是由于气氛中CO2同时参与了还原反应的逆反应:
ZnFe2O4-δ+CO2→ZnFe2O4+C (7)
Fe3O4-δ+CO2→Fe3O4+C (8)
CO2可修复铁酸锌中CO还原形成的氧缺位[9-10],逆反应的存在使铁酸锌的失氧逐渐达到平衡,还原反应受限。
750 ℃下等温还原产物中Fe2+和ZnO含量随反应时间的变化曲线如图4(a)所示,由图4(a)可见,从0到40 min还原生成的ZnO和Fe2+含量(Fe2+占总铁的质量分数w(Fe2+))分别从1.19%、1.51%大幅增加至68.49%和24.83%,之后ZnO和Fe2+含量仍微弱增加,最高可达120 min的72.35%和25.88%。反应过程中产物的Fe2+含量均未超过磁铁矿中的Fe2+含量(33.33%),说明该反应条件抑制了Fe2+的过量生成。ZnO和Fe2+的变化趋势相同,ZnO含量随Fe2+的增加而增加。采用Origin对ZnO和Fe2+含量进行线性拟合,拟合结果如图4(b)所示。由图4(b)可得ZnO和Fe2+含量存在显著的线性关系,回归方程为w(ZnO)=2.8014w(Fe2+)+ 0.32113 (R2=0.98822),w(ZnO)与w(Fe2+)分别表示ZnO含量和Fe2+含量。由此可见,ZnO和Fe2+含量密切相关,两者基本符合反应(1)方程式中ZnO与Fe2+含量3倍的关系。结合质量损失分析可得,铁酸锌的还原是由失氧引起的,部分铁因维持电中性还原为二价,Fe2+则促使铁酸锌分解产生ZnO,两者之间符合线性关系。
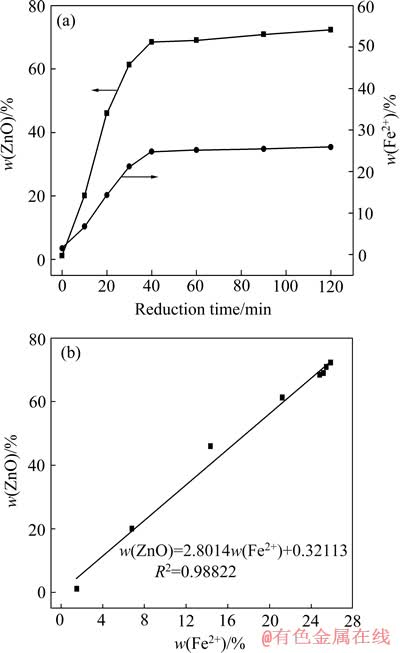
图4 不同时间还原产物的ZnO与Fe2+含量及其线性拟合曲线
Fig. 4 Mass fraction of ZnO and Fe2+ of reduced samples with different times(a) and linear fitting curve of mass fraction of zinc oxide and Fe2+(b)
2.3 离子迁移行为
2.3.1 Zn 2p3/2 峰分析
不同还原时间还原产物的Zn 2p3/2 XPS谱如图5所示。由图5可见,原样品中Zn 2p3/2结合能为1021.27 eV,与文献[15]中报道的铁酸锌中锌的结合能一致。还原5 min后,Zn 2p3/2结合能增加到1021.60 eV,峰位和峰宽与文献[17]中ZnO的值一致。结合能增加是由于还原反应失氧,使表面电子密度降低,屏蔽效应减弱,同时也说明5 min内ZnO已在表面形成。
还原反应前后Zn 2p3/2的半峰宽有所变窄,从1.99 eV降低为1.57 eV,说明Zn2+的赋存状态发生了改变。但峰型对称,表明原样和还原样品中表面Zn2+的化学环境和原子占位都是均一的。由于Zn2+在正尖晶石和ZnO中都占据于四面体位置,而八面体占位的Zn2+[16](ZnTiO3, 1023.0 eV)结合能较高,因此,经过长时间的退火处理,原样品和还原产物中的Zn2+均占据于四面体位置,并未发生原子占位的混乱,铁酸锌表面锌的还原产物为ZnO。
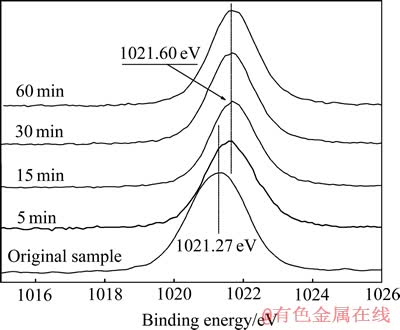
图5 原样及还原产物的Zn 2p3/2的XPS谱
Fig. 5 XPS spectra of Zn 2p3/2 of original sample and reduced samples with different times
随着反应进行到60 min,Zn 2p3/2的峰位和峰宽与5 min时相比均未发生较大变化。还原反应虽在表面持续进行,但并不影响表面锌原子的化学态,Zn2+仍以ZnO的形式存在。在选择性还原条件下,气固反应界面在ZnO形成后结构能保持相对稳定,避免了ZnO还原导致Zn2+占位混乱,固溶至铁氧化物中使锌回收率降低。
2.3.2 Fe 2p 峰分析
不同还原时间还原产物的Fe 2p的XPS谱如图6所示。在图6中两个显著的特征峰分别对应于Fe 2p1/2和Fe 2p3/2。原样中Fe 2p3/2结合能为711.34 eV,文献[22]中Fe2+和Fe3+的2p3/2结合能分别为709.5 eV和711.6 eV,可见原样品中的铁主要以三价铁的形式存在。还原5 min后,Fe 2p3/2 的峰位为711.14 eV,与原样品相比,向低能位有轻微的偏移。随着反应的继续进行,Fe 2p峰与Zn 2p3/2一样未发明显变化,进一步证实了选择性还原气氛中表面铁的性质同样相对稳定。
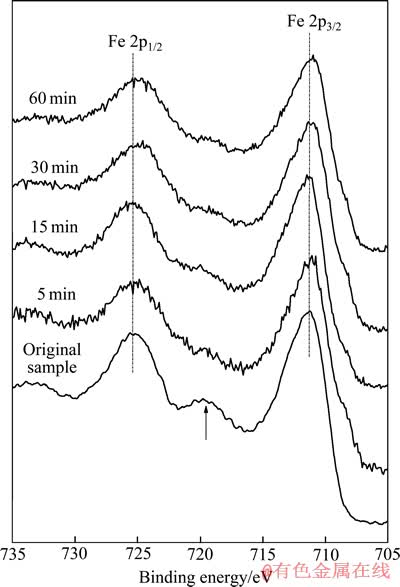
图6 原样品及还原产物的Fe 2p的XPS谱
Fig. 6 XPS spectra of Fe 2p of original sample and reduced samples with different times
Fe2+和Fe3+会分别产生高于Fe 2p3/2主峰强度8 eV和6 eV的震激峰[23]。由原样的XPS谱可见,箭头所指的卫星峰峰值为919.9 eV,大约高于Fe 2p3/2主峰峰值(8.4 eV),这是由尖晶石中全部的Fe3+产生的。但是经过5 min还原反应之后,Fe 2p1/2和2p3/2峰之间变得相对平坦,行星峰消失,Fe 2p峰峰型与文献[23]中磁铁矿的峰型一致,这是由还原产生的Fe2+与Fe3+的震激峰重叠导致的。结合Zn 2p峰的分析结果可得,还原反应5 min后,ZnO和磁铁矿同时在表面形成,Fe2+取代了铁酸锌尖晶石晶格中的Zn2+,ZnO从铁酸锌中析出,而磁铁矿则在尖晶石中形成,还原产物ZnO与磁铁矿之间互不固溶。
不同还原时间还原产物的表面摩尔如图7所示。由图7可见,还原5 min后,表面锌含量大幅增加,同时铁含量相对减少,n(Zn)/n(Fe)增加到1.84,远超过铁酸锌中的n(Zn)/n(Fe)。说明还原过程中存在铁锌离子的相对迁移,Fe2+从表面向内部迁移,Zn2+被Fe2+取代向外部扩散并富集于表面,锌的过饱和使ZnO在表面形核,同时,Fe2+占据尖晶石内部中八面体间隙形成磁铁矿。
随着反应的进行,离子的迁移距离逐步增长,使得反应速率持续降低,ZnO从表面生长转变为垂直生长[14],因此,表面锌的含量逐渐降低,而表面铁的含量则逐渐增加。在整个反应过程中,氧的相对含量则保持基本恒定,在持续质量损失的还原反应中,表面应为连续失氧,这说明表面氧有内部的氧向外迁移作为补充,同时气氛中的CO2也可修复过量的氧空位,防止过量失氧造成的表面磁铁矿过还原。
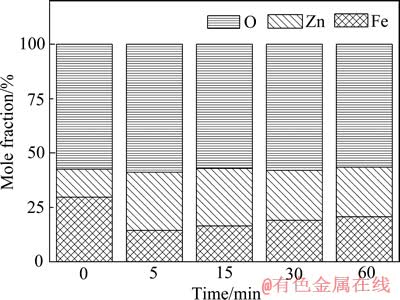
图7 原样及还原产物的表面摩尔分数
Fig. 7 Mole fraction of surface of original sample and reduced samples with different times
2.4 物相转变过程
不同还原时间下还原产物的XRD谱如图8(a)所示。由图8(a)可见,物相成分主要包括ZnO、铁酸锌和磁铁矿。10 min后,衍射角31.7°和36.2°处对应于氧化锌的衍射峰开始出现;20 min后,ZnO的特征峰变得明显;30 min后,峰强显著增强,之后,图谱中各峰强度变化不大。在整个还原过程中,尖晶石的峰一直存在,不能分辨出单独的铁酸锌和磁铁矿。XPS的分析结果说明,确有磁铁矿生成,但由于铁酸锌和磁铁矿结构相同形成了连续固溶体,因此,在XRD谱中不能检出磁铁矿的峰,但可以根据尖晶石特征峰峰位的偏移和晶胞参数的变化说明尖晶石的物相转变过程。
图8(b)所示为还原产物60°~65°的衍射谱。由图8(b)可见,尖晶石(440)衍射峰峰型尖锐,结晶良好,说明在还原过程中尖晶石结构十分稳定。20 min后,ZnO的衍射峰在(440)峰的右侧形成,峰强逐步增强,(440)衍射峰的强度则未发生显著变化,但峰位先向左偏后向右移。衍射峰峰位的偏移反映出了还原过程中尖晶石的晶格存在膨胀和收缩。
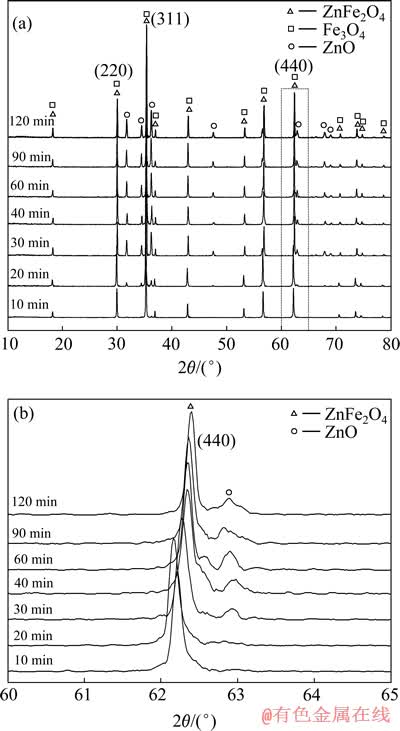
图8 不同时间还原产物的XRD谱
Fig.8 XRD patterns of reduced samples with different times
科恩最小二乘法计算的还原产物晶胞参数如表1所列,其变化趋势如图9所示,由图9可见,未反应铁酸锌的晶胞参数为8.437  ,20 min后,晶胞参数增加至8.441
,20 min后,晶胞参数增加至8.441  ,之后逐渐降低,直至降为40 min的8.419
,之后逐渐降低,直至降为40 min的8.419  。物相转变是由离子迁移所造成的,因此,反映还原相变过程的晶胞参数的变化应归因于Fe2+的迁移和Zn2+的取代。
。物相转变是由离子迁移所造成的,因此,反映还原相变过程的晶胞参数的变化应归因于Fe2+的迁移和Zn2+的取代。
表1 不同时间还原产物中尖晶石相的晶胞参数
Table 1 Lattice parameters of spinel phase in reduced samples with different times

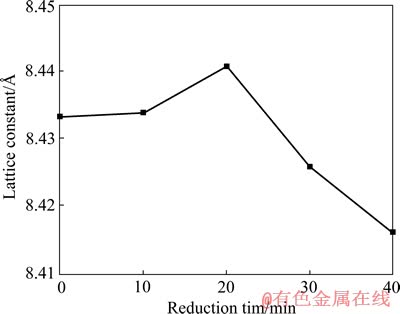
图9 不同时间还原产物中尖晶石相的晶胞参数
Fig. 9 Lattice parameter of spinel phase in reduced samples with different times
还原生成的Fe2+倾向于占据尖晶石中的八面体间隙,因而会迁移到尖晶石内部去取代Fe3+占据的八面体间隙,或者直接占据空余的八面体空隙。Fe2+和替代的锌铁离子在晶格当中的嵌入和占位会使晶格膨胀、晶胞参数增大,因此,反应0到20 min时,晶胞参数增大。Fe2+的原子半径较Zn2+的小,随着尖晶石中Zn2+被Fe2+持续的替代,Zn2+从尖晶石中迁出,会使晶格收缩,晶胞参数降为8.419  ,但仍大于文献中[13]磁铁矿的晶胞参数值,说明仍有部分铁酸锌未还原,与还原产物磁铁矿固溶形成了含锌磁铁矿。此时的还原产物应为从铁酸锌中析出的ZnO和含锌磁铁矿。
,但仍大于文献中[13]磁铁矿的晶胞参数值,说明仍有部分铁酸锌未还原,与还原产物磁铁矿固溶形成了含锌磁铁矿。此时的还原产物应为从铁酸锌中析出的ZnO和含锌磁铁矿。
2.5 产物层形貌变化
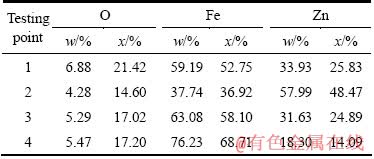
铁酸锌及90 min还原反应后样品的扫描电镜图如图10所示。由图10可见,原样颗粒性质均一,粒径约为70 μm左右,颗粒内部分布有不规则孔隙。表2的能谱定量分析表明原样颗粒点1处n(Zn)/n(Fe)约为1/2,说明原样为标准的铁酸锌。还原反应90 min后,产物颗粒大小基本未变,但形貌发生了大幅变化,变成深浅夹杂的产物层包裹着浅色的未反应核。产物层中点2和点4的n(Zn)/n(Fe)均偏离了1/2,其中点2处富锌,点4处富铁,说明还原样品中的铁锌离子发生了扩散迁移,使产物层中发生了铁锌元素的局部富集。点2处主要为ZnO,点4处则主要为磁铁矿,还原产物磁铁矿和ZnO并非单体解离,而是相互嵌布。
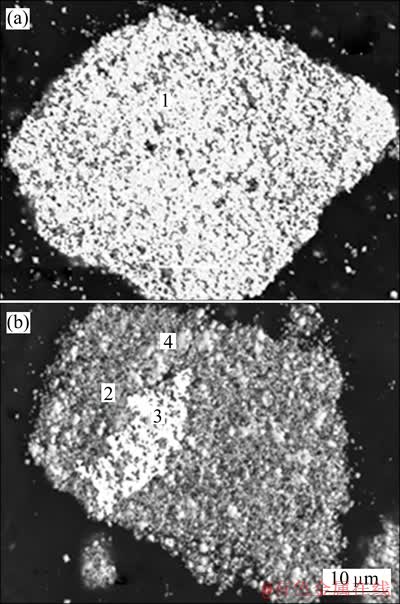
图10 原样及还原样品的SEM像
Fig. 10 SEM images of original sample(a) and reduced sample(b)
表2 图10中不同测试点元素组成
Table 2 Elements component of points 1-4 shown in Fig. 10
SEM-EDS的分析进一步证实了铁酸锌的还原产生了离子的迁移与扩散,并且直接观测到未反应的铁酸锌核。
3 结论
1) 热力学优势区域图分析表明,750 ℃下,铁酸锌的选择性还原需控制φ2(CO)在2.68%~36.18%之间,当φ1(CO)=8%和φ2(CO)=20%时,能够实现选择性还原。
2) 质量损失与分解的过程趋势相同,反应最终都达到平衡,此时的质量损失低于理论质量损失,选择性反应条件避免铁酸锌的过还原。反应表现为失氧过程,还原产生的Fe2+使铁酸锌分解产生ZnO,因此,ZnO含量与Fe2+含量线性相关,选择性还原条件下的回归方程为w(ZnO)=2.8014w(Fe2+)+0.32113。
3) 离子迁移行为包括Fe2+向尖晶石内部的迁移,Zn2+被Fe2+所替代,向外迁移并富集于表面,促使ZnO在表面的形核,磁铁矿同时在表面生成,O2-从内部向表面迁移以弥补表面失氧。
4) 物相转变过程是ZnO从铁酸锌中析出,而铁酸锌转变为磁铁矿的过程,相变过程中由于铁锌离子的迁移和替代,出现了晶胞参数先增大后减小的变化,还原产物为ZnO和含锌磁铁矿。
5) 产物层的形貌变化说明了还原过程中的离子迁移与扩散导致了产物层中铁锌的局部富集,还原产物相互夹杂嵌布,包裹着未反应的铁酸锌核。
REFERENCES
[1] LI Mi, PENG Bing, CHAI Li-yuan, PENG Ning, YAN Huan, HOU Dong-ke. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon[J]. Journal of Hazardous Materials, 2012, 238(10): 323-330.
[2] 王纪明, 彭 兵, 柴立元, 李 密, 彭 宁. 锌浸渣还原焙烧-磁选回收铁[J]. 中国有色金属学报, 2012, 22(5): 1455-1461.
WANG Ji-ming, PENG Bing, CHAI Li-yuan, LI Mi, PENG Ning. Recovery iron from zinc leaching residues by reduction roasting and magnetic separation process[J]. The Chinese Journal ofNonferrousMetals, 2012, 22(5): 1455-1461.
[3] 韩俊伟, 刘 维, 覃文庆, 柴立元, 郑永兴, 杨 康. 高铁锌焙砂选择性还原焙烧-两段浸出锌[J]. 中国有色金属学报, 2014, (2): 511-518.
HAN Jun-wei, LIU Wei, QIN Wen-qing, CHAI Li-yuan, ZHENG Yong-xing, YANG Kang. Leaching zinc from high iron-bearing zinc calcine after selective reduction roasting[J]. TheChineseJournalofNonferrousMetals, 2014, (2): 511-518.
[4] Raghavan V. Fe-O-Zn (iron-oxygen-zinc)[J]. Journal of Phase Equilibria and Diffusion, 2010, 31(4): 373-376.
[5] 梁美生. 铁酸锌高温煤气脱硫行为及气氛效应研究[D]. 太原: 太原理工大学, 2005.
LIANG Mei-sheng. The desulfurization behaviour of ZnFe2O4 sorbent in hot gas clean-up and the effects of gas atmosphere on this process[D]. Taiyuan: Taiyuan University of Technology, 2005.
[6] Gheisari M, Mozaffari M, Acet M, Amighian j. Preparation and investigation of magnetic properties of wüstite nanoparticles[J]. Journal of Magnetism and Magnetic Materials, 2008, 320(21): 2618-2621.
[7] 李 密. 锌焙砂选择性还原与铁锌分离的基础研究[D]. 长沙: 中南大学, 2013.
LI Mi. Fundametal reserach on selective reduction of zinc calcine and separation of zinc and iron[D]. Changsha: Central South University, 2013.
[8] O'Neill H S C, Navrotsky A. Simple spinels; crystallographic parameters, cation radii, lattice energies, and cationdistribution[J]. American Mineralogist, 1983, 68(1/2): 181-194.
[9] 傅毛生, 陈林深, 李建国, 陈诵英. 还原条件对 NiFe2O4-δ结构稳定性及其催化分解CO2活性的影响[J]. 燃料化学学报, 2007, 35(4): 431-435.
FU Mao-sheng, CHEN Lin-shen, LI Jian-guo, CHEN Song-ying. Effect of reduction condition on structure stability of NiFe2O4-δ and its catalytic performance of CO2 decomposition[J]. Journal of Fuel Chemistry and Technology, 2007, 35(4): 431-435.
[10] 张春雷, 吴通好, 杨洪茂, 姜玉子. 氧缺位的磁铁矿型化合物转化CO2成C的研究[J]. 无机化学学报, 1996, 12(1): 61-66.
ZHANG Chun-lei, WU Tong-hao, YANG Hong-mao, JIANG Yu-zi. Studies on the conversion of carbon dioxide into carbon over the oxygen-deficient magnetite[J]. Journal Inorganic Chemistry, 1996, 12(1): 61-66.
[11] 朴玲钰, 李春虎, 李彦旭. ZnFe2O4 高温煤气脱硫剂的还原与硫化[J]. 高校化学工程学报, 2001, 15(6): 546-551.
PIAO Ling-yu, LI Chun-hu, LI Yan-xu. Study on reduction and sulfidation of ZnFe2O4 desulfurizer at high temperature[J]. Journal of Chemical Engineering of Chinese Universities, 2001, 15(6): 546-551.
[12] Kodama T, Tabata M, Sano T, TSUJI M, TAMAURA Y. XRD and  studies on oxygen-deficient Ni(Ⅱ)-bearing ferrite with a high reactivity for CO2 decomposition to carbon[J]. Journal of Solid State Chemistry, 1995, 120(1): 64-69.
studies on oxygen-deficient Ni(Ⅱ)-bearing ferrite with a high reactivity for CO2 decomposition to carbon[J]. Journal of Solid State Chemistry, 1995, 120(1): 64-69.
[13] Makovec D, Drofenik M. Non-stoichiometric zinc-ferrite spinel nanoparticles[J]. Journal of Nanoparticle Research, 2008, 10(1): 131-141.
[14] Tong L F. Reduction mechanisms and behaviour of zinc ferrite—Part 1: Pure ZnFe2O4[J]. Mineral Processing and Extractive Metallurgy, 2001, 110(1): 14-24.
[15] Bera S, Prince A A M, Velmurugan S, RAGHAVAN P S, GOPALAN R, PANNEERSELVAM G, NARASIMHAN S V. Formation of zinc ferrite by solid-state reaction and its characterization by XRD and XPS[J]. Journal of materials science, 2001, 36(22): 5379-5384.
[16] Druska P, Steinike U,  V. Surface structure of mechanically activated and of mechanosynthesized zinc ferrite[J]. Journal of Solid State Chemistry, 1999, 146(1): 13-21.
V. Surface structure of mechanically activated and of mechanosynthesized zinc ferrite[J]. Journal of Solid State Chemistry, 1999, 146(1): 13-21.
[17]  V, Steinike U, Uecker D C,
V, Steinike U, Uecker D C,  S, BECKER K D. Structural disorder in mechanosynthesized zinc ferrite[J]. Journal of Solid State Chemistry, 1998, 135(1): 52-58.
S, BECKER K D. Structural disorder in mechanosynthesized zinc ferrite[J]. Journal of Solid State Chemistry, 1998, 135(1): 52-58.
[18] 张燕娟, 黎铉海, 潘柳萍, 韦岩松. 机械活化对铟铁酸锌溶解动力学及物化性质的影响[J]. 中国有色金属学报, 2012, 22(1): 315-323.
ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, WEI Yan-song. Influences of mechanical activation on dissolution kinetics and physicochemical properties of indium-bearing zinc ferrite[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 315-323.
[19] 张惠斌. 矿石和工业产品化学物相分析[M]. 北京: 冶金工业出版社, 1992: 138-352.
ZHANG Hui-bin. Chemical phase analysis of ores and industrial products[M]. Beijing: Metellurgical Industry Press, 1992: 138-352.
[20] 夏秀文, 何 维, 黄津梨. 最小二乘法精确测定Gd3Co1-xVx的点阵常数和固溶度[J]. 稀土, 2009, 30(1): 56-60.
XIA Xiu-wen, HE Wei, HUANG Jin-li. Accurate measurement of lattice constants and vanadium solid solubility for Gd3Co1-xVx compounds by least square method[J]. Chinese Rare Earths, 2009, 30(1): 56-60.
[21] 梁敬魁. 粉末衍射法测定晶体结构[M]. 北京: 科学出版社, 2003: 340-342, 540-542.
LIANG Jing-kui. Crystal structure determination by X-ray powder diffraction[M]. Beijing: Science Press, 2003: 340-342, 540-542.
[22] Mills P, Sullivan J L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy[J]. Journal of Physics D: Applied Physics, 1983, 16(5): 723.
[23] Yamashita T, Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials[J]. Applied Surface Science, 2008, 254(8): 2441-2449.
(编辑 李艳红)
基金项目:国家高技术研究发展计划资助项目(2011AA061000)
收稿日期:2014-01-20;修订日期:2014-05-12
通信作者:彭 兵,教授,博士;电话:0731-88830577;E-mail: pb@csu.edu.cn