J. Cent. South Univ. (2017) 24: 1082-1089
DOI: 10.1007/s11771-017-3511-z
Comprehensive recovery of Fe, Zn, Ag and In from high iron-bearing zinc calcine
PENG Bing(彭兵)1, 2, PENG Ning(彭宁)1, LIU Hui(刘恢)1, 2, XUE Ke(薛珂)1, LIN Dong-hong(林冬红)1
1. Institute of Environmental Science and Engineering, School of Metallurgical Science and Engineering,
Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Centre for Control & Treatment of Heavy Metal Pollution,
Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: A beneficiation-metallurgy combination process is developed to recover Zn, Fe and to enrich In, Ag from high iron-bearing zinc calcine based on our former researches. In gaseous reductive roasting process, the roasting conditions were tested by magnetic separation of roasted product. It is found that the VCO (PCO/(PCO+CO2) in roasting atmosphere should be maintained below 30% to avoid the generation of zinc iron solid solution (Fe0.85-xZnxO), which can bring a decrease of iron recovery in magnetic separation. After roasting, acid leaching and multistage magnetic separation are carried out for the recovery of Zn, Fe and enrichment of Ag and In. About 90% of zinc is extracted and 83% of iron is recovered in the whole process. The Ag mainly enters the tailings with a recovery of 76%, the Ag grade increases from 0.12 g/t in raw materials to 1.18 g/t in the tailings. However, the In mainly enters the iron concentrations and the recovery reaches 86%. This process was proved to be technically feasible and may be a favorable option in the treatment of high iron-bearing zinc material with high Ag or In content.
Key words: high iron-bearing zinc calcine; reductive roasting; multistage magnetic separation; silver recovery; indium recovery
1 Introduction
Traditional roasting-leaching-electrowinning process in zinc production generates a significant amount of high iron- and zinc- bearing residue. Zinc ferrite, a franklinite, is formed in desulfurization roasting of high iron-bearing zinc sulfide, which causes losses of metal value and serious heavy metal pollution in stockpile and transportation.
Beacuse of the comprehensive utilization of metal values, such as Zn, Fe, Pb, Ag, In, and the reduction of residues, it is necessary to separate zinc and iron by decomposing zinc ferrite in high iron-bearing zinc calcine. Many processes such as Waelz and Ausmelt were developed according to the different boiling points of zinc and iron. In these processes, zinc is volatilized at high temperature above 1100 oC and recovered in fume dust as zinc oxide, iron is remained in kiln residues [1-3]. However, these pyro-metallurgical processes were energy intensive [4, 5]. On the other hand, hydrometallurgical processes such as hot acid leaching- iron precipitation process [6-8] and many lab-scale processes [9, 10] decomposed zinc ferrite into zinc and iron ions. The purification of zinc solution is obligatory to remove iron by raising pH value. Even though these hydrometallurgical processes will product large amounts of iron precipitation residues, they consume much less energy than pyro-metallurgical processes. However, the residues generate a significant environmental concern [11].
Some decomposition-leaching methods were proposed for the treatment of zinc ferrite after the consideration above. The zinc ferrite in calcine is first roasted at relatively low temperature to make a desirable phase change, and then the roasted product is subjected to zinc leaching. These methods could be classified into carbonate roasting-leaching [12-14], sulfate roasting- leaching [15] and chloride roasting-leaching [16]. But all of them focus on the recovery of zinc and left iron in the residues.
In our previous research, a reductive roasting and magnetic dressing process was developed [17-19]. However, the elements balance including Pb, Ag, In in the whole roasting-leaching-magnetic separation process is still not known and further research on
multistage magnetic separation is still needed. The objective of this study is to selectively decompose zinc ferrite in high iron-bearing zinc calcine into zinc oxide and magnetite without the generation of ferrous oxide, accumulating elements such as Ag, Pb and In accompanied with the separation of Zn and Fe. A mixture of CO and CO2 is used as reductant in reductive roasting, and then the roasted product is subjected to acid leaching and multistage magnetic separation. The parameters of reductive roasting and the elements balance of overall process are determined.
2 Materials and methods
2.1 Materials
The zinc calcine used in this study was obtained from a zinc plant of Hunan, China. It is a kind of taupe-colored powder whose stockpiling density and angle of repose were 1.7 g/cm3 and 45°, respectively. The sample was dried at 105 °C for 24 h before the experiments. The SEM-EDS spectra of zinc calcine are given in Fig. 1. The main elements in zinc calcine are Zn, Fe, O, S, and the minor elements Mn, Cu, Ca appeared in area III. The SEM-EDS spectrum revealed zinc and iron sintered together in the grain. Also, the existence of element sulfur in area II shows that the zinc calcine was not sufficiently desulfurized, which would bring difficulties for the further zinc recovery from reductive roasting products. An ICP-AES analysis of zinc calcine (Table 1) shows that Zn, Fe, S, Pb and Cu are the main elements. The silver content of zinc calcine is 141 μg/g, which is worthy of being enriched by magnetic separation.
2.2 Methods
1) Reductive roasting
The roasting process was conducted in a customized tube furnace designed by ourselves (Fig. 2). N2, CO and CO2 were mixed at a certain ratio prior to pump into furnace. Samples were heated under N2 flow at a certain flow rate and then replaced by reductive gas mixture once the pre-set reduction temperature achieved. After a certain roasting duration, the hot samples were taken out by descending the elevator and quenched by water. The factors affect the decomposition rate of zinc ferrite in roasting process including roasting temperature (650-800 °C/ at 50 °C/min), roasting duration (15- 120 min), PCO (4%-8%) and VCO (10%-40%) were examined.
2) Magnetic separation
Davis tube tester and slurry of reduced samples (solid 10%) were applied in MS (magnetic separation). The magnetic field intensity ranging from 200 to 3000 Gs in the center of gap between poles was controlled by modifying current value [18]. After a certain duration, the electromagnet was switched off and the concentration was washed with 2-3 L water. The magnetic fractions separated by Davies tube were filtered,
dried, weighed and sent for analysis. The recovery of target element (such as iron and zinc) was calculated in following equation.
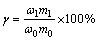
where γ is recovery; ω1 is grade of target element in concentration; m1 is mass of concentration; ω0 is grade of target element in raw materials; m0 is mass of raw material.

Fig. 1 SEM image of zinc calcine (a) and its EDS spectra (b, c, d)
Table 1 ICP-AES analysis of zinc calcine
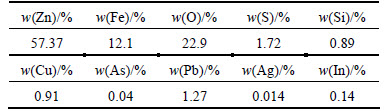
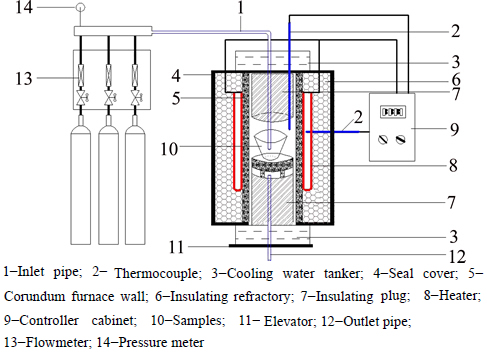
Fig. 2 Schematic plot of tube furnace:
3) Acid leaching
Acid leaching was carried out with sulfuric acid as leaching agent in a 1-L conical flask heated by water bath equipped with a thermostatic thermocouple and a mechanical stirrer controlled at a certain speed. The leaching conditions were set according to our preliminary work finished by WANG [20], leaching temperature 30 °C, acid concentration 90 g/L and time duration 20 min, stirring speed 400 r/min and L/S ratio 10:1.
4) Characterization
The grades of iron and zinc were analyzed by permanganate titration and EDTA titration respectively. ICP-AES (Baird, PS-6) was used for the analysis of Pb, S, Ag and In in samples. Hysteresis loops of raw sample and roasted product were characterized by vibrating sample magnetometer (Model: HH-15). SEM-EDS analysis (JEOL, Ltd., JSM-6360LV; EDAX, Ltd, EDX-GENESIS 60S) was applied in the microstructure characterization of samples.
3 Results and discussion
3.1 Effect of roasting parameters on magnetic separation
The effect of roasting parameters on magnetic separation (MS) was investigated when the roasted product was separated under magnetic field intensity of 930 Gs for 10 min. Figure 3 shows that the iron recovery and grade in MS as a function of various roasting parameters including CO concentration, PCO (Figs. 3(a) and 3(b)), roasting temperature (Figs. 3(c) and 3(d)) and PCO/(PCO+PCO2) (Figs. 3(e) and (f)). Conditions of each figures are listed in Table 2. Figures 3(a) and (b) show that the iron recovery of the roasted product increases faster at a higher PCO, and PCO does not influence the iron recovery notably when it exceeds 6%, and the highest iron recovery is achieved after being roasted for 30 min under PCO 8%. Figures 3(c) and (d) show that recovery of iron of roasted product increases rapidly with the prolong of roasting time when the roasting temperature is below 750 oC, which reveals that the reaction rate is elevated with the increase of roasting temperature when roasted at 650-750 °C. But the influence of roasting temperature levels off on the reaction rate when it is above 750 °C. To save energy, we select 750 °C as the optimal roasting temperature for the next series of experiments. The effect of VCO was studied and the results are shown in Figs. 3(e) and (f). Iron recovery decreases after roasting for 90 min under VCO 40%. When VCO reaches 100% (without CO2), the decrease of recovery becomes obvious compared to other curves. The zinc ferrite may be seriously over-reduced form FeO and ZnO at high VCO, consequently, the decrease of iron recovery is caused. Also, it is noted that the grade of iron in concentration is generally below 20%. This phenomenon reveals that separation of iron from roasted product is hardly to achieve only by magnetic separation.
3.2 Characterization of roasted zinc calcines
Figure 4 shows the particle size distribution of samples before and after reductive roasting. An increase of particle size is observed due to the sintering of samples, which indirectly demonstrates the generation of iron oxide with the decomposition of zinc ferrite as reported by EL-SHOBAKY and RADWAN [21].
The SEM-EDS spectrum of roasted product under the optimal roasted conditions is displayed in Fig. 5. The image shows a loose structure with an outer layer of zinc compound (B area) for grains of roasted zinc calcine. Chemical analyses of area A and area C reveal complicated phases composed by Zn, Fe, Ca, Mn or Zn, S, Fe, respectively. The existence of these phases brings
difficulty to the direct separation of iron by one stage magnetic separation.
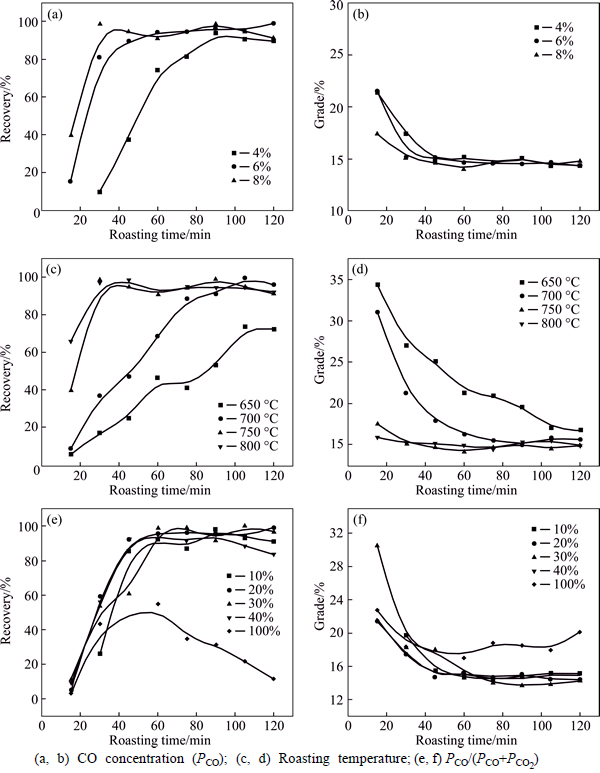
Fig. 3 Effects of roasting parameters on magnetic separation: (a, b) CO concentration (PCO); (c, d) Roasting temperature;(e, f) PCO/(PCO+PCO2)
Table 2 Roasting conditions for Fig. 3
In Fig. 3(e), the iron recovery decreases when the VCO increases above 40%. To find out the magnetic property of samples roasted under the different VCO, magnetic hysteresis loops of the samples were carried out and the results are shown in Fig. 6(a). The value of Bm presents the value of saturation magnetization of materials. In magnetic separation, the increase in Bm of the roasted product favors the separation process. Thus, the target of reductive roasting is to get the roasted product with a higher Bm. However, a decrease of the Bm value is found from these curves in Fig. 6(a) with the increase of the VCO, which is in consistent with the
results of magnetic separation. It can be seen from the XRD spectra of roasted samples gained under the different VCO that Fe0.85-xZnxO was generated at the VCO over 30%. The existence of Fe0.85-xZnxO proves the over-reduction of Fe3O4 as Fe0.85-xZnxO is generated by FeO and ZnO. So, it is suggested that the VCO in roasting process should be maintained below 30%.

Fig. 4 Effects of roasting parameters on magnetic separation:
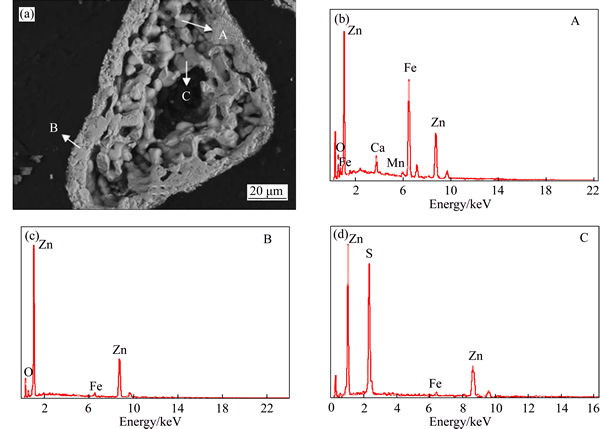
Fig. 5 SEM image (a) and EDS spectra (b, c, d) of roasted zinc calcine

Fig. 6 Hysteresis loop (a) and XRD spectra (b) of roasted zinc calcine
3.3 Element balance of leaching-multistage magnetic separation
The leaching rates of iron and zinc under the optimal leaching conditions are shown in Table 3. About 90% of zinc and 6% of iron are leached. The iron grade in the leaching residues is 42.1%. To design the multistage magnetic separation, we investigated the effect of magnetic field intensity on the recovery and grade of iron. Figure 7 shows that yield and recovery, grade of iron of the leaching residues as a function of magnetic intensity. Each value of magnetic intensity was selected according to the magnetizing current value which could be modified easily. With the increase of magnetic intensity, yield and iron recovery elevate rapidly and reach their peak value at an intensity of 480 Gs, and then their just increase slowly. However, the iron grade decreases continuously in the series of experiments.
A leaching-multistage magnetic separation process was designed to recover zinc and iron from roasted zinc calcine and enrich Ag, In and Pb in the tailings of magnetic separation based on above study. The overall flow sheet of multistage magnetic separation is a and the magnetic intensity in each stage of magnetic separation are shown in Fig. 8. Iron rich concentration and zinc rich tailing were finally got and the main elements balance in whole process is displayed in Table 3.
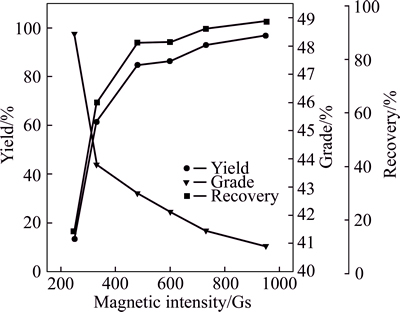
Fig. 7 Magnetic separation results of leaching residues
1) Zn and Fe
After leaching of roasted zinc calcines, the zinc mass ratios in leaching solution and residues are 90.07% and 9.93%, respectively. It is reasonably deduced that the main zinc phase in leaching residue is ZnS and oxygen-deficient zinc ferrite (ZnFe2O4-x). In the following magnetic separation, the zinc presented in iron concentration is mainly ZnFe2O4-x as sulfur content is low [22]. Iron concentration is obtained under iron
recovery 83.24% and iron grade 50.90%, which could be applied in the bath smelting process of lead as fluxing medium.

Fig. 8 Multistage magnetic separation (a) and whole flow sheet adopted in this study (b)
Table 3 Elements balance of whole process

2) In, Pb and Ag
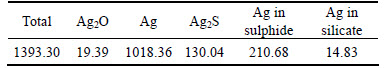
In leaching procedure, only a little In was leached out. In the next multistage magnetic separation, Pb was hard to be separated so that the distribution of it in iron concentration and zinc tailings was almost the same. However, Ag mainly entered into the zinc tailings of magnetic separation in grade 1.18 g/t under recovery 75.96%; Table 4 lists the phase composition of silver in tailings. It can be seen that silver mainly exists as silver metals. The variation between silver grade in Table 3 and Table 4 was caused by the difference between the ICP analysis and chemical analysis. This part of silver could be recovered by flotation process as reported by ZHOU [23]. Though there are errors in the results of In content, it reveals a trend that In mainly exists in iron concentration with a recovery of 85.78% for In easily enters the spinel lattice of Fe3O4 or ZnFe2O4-x to form a kind of solid solution [24, 25]. Utilization of these iron concentrations as auxiliary material in the bath smelting process of lead may be a potential option to recovery these In.
Table 4 Phase composition of Ag in tailings of magnetic separation (g/t)
4 Conclusions
1) 100% of iron recovery is obtained in magnetic separation after roasting at 750 °C for 45-60 min under PCO 6%-8% and VCO 20%-30 %.
2) Fe0.85-xZnxO is generated at the VCO higher than 30% and it causes a decrease of iron recovery in magnetic separation.
3) Fe and Zn are recovered separately in leaching-multistage magnetic separation. Ag is enriched in the tailings with a recovery of 75% and a grade of 1.18 g/t, and about 79% of In enters into the iron concentration.
References
[1] LI Mi, PENG Bing, CHAI Li-yuan, PENG Ning, YAN Huan, HOU Dong-ke. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon [J]. Journal of Hazardous Materials, 2012, 237: 323-330.
[2] KOSSEK G, SCHNABEL W, REUTER G, SERBENT H. Waelz process of volatilizing zinc and lead from iron oxide-containing materials: US, 4238222 [P]. 1980.
[3] SERBENT H, REUTER G, SCHNABEL W, KOSSEK G. Removal of zinc and lead from materials containing iron oxide by the rotary-kiln volatilizing process: EP, 7662 [P]. 1980.
[4]  M, PEKDEMIR T,
M, PEKDEMIR T,  S, KNK L A. Industrial symbiosis: High purity recovery of metals from Waelz sintering waste by aqueous SO2 solution [J]. Journal of Hazardous Materials, 2007, 149(2): 303-309.
S, KNK L A. Industrial symbiosis: High purity recovery of metals from Waelz sintering waste by aqueous SO2 solution [J]. Journal of Hazardous Materials, 2007, 149(2): 303-309.
[5] BESE A V, BORULU N, COPUR M, COLAK S, ATA O N. Optimization of dissolution of metals from Waelz sintering waste (WSW) by hydrochloric acid solutions [J]. Chemical Engineering Journal, 2010, 162(2): 718-722.
[6] FFILIPPOU D, DEMOPOULOS G. Steady-state modeling of zinc-ferrite hot-acid leaching [J]. Metallurgical and Materials Transactions B, 1997, 28(4): 701-711.
[7] FILIPPOU Dimitrios, DEMOPOULOS George. A reaction kinetic model for the leaching of industrial zinc ferrite particulates in sulphuric acid media [J]. Canadian Metallurgical Quarterly, 1992, 31(1): 41-54.
[8] ELGERSMA F, KAMST G F, WITKAMP G J, van ROSMALEN G M. Acidic dissolution of zinc ferrite [J]. Hydrometallurgy, 1992, 29(1): 173-189.
[9] FARAHMAND F, MORADKHANI D, SAFARZADEH M S, RASHCHI F. Brine leaching of lead-bearing zinc plant residues: Process optimization using orthogonal array design methodology [J]. Hydrometallurgy, 2009, 95(3, 4): 316-324.
[10] LI Cun-xiong, WEI Chang, FAN Gang. Pressure acid leaching of high silicon zinc oxide ore [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(9): 1678-1683. (in Chinese)
[11] STOPIC S, FRIEDRICH B. Kinetics and mechanism of thermal zinc-ferrite phase decomposition [C]// Proceeding of EMC. Aachen, 2009, 28: 1167-1181.
[12] HOLLOWAY P C, ETSELL T H., MURLAND A L. Use of secondary additives to control the dissolution of iron during Na2CO3 roasting of La oroya zinc ferrite [J]. Metallurgical and Materials Transactions B, 2007a, 38(5): 793-808.
[13] HOLLOWAY P C, ETSELL T H, MURLAND A L. Roasting of La Oroya zinc ferrite with Na2CO3 [J]. Metallurgical and Materials Transactions B, 2007, 38(5): 781-791.
[14] HOLLOWAY P C, ETSELL T H. Recovery of zinc, gallium and indium from La Oroya zinc ferrite using Na2CO3 roasting [J]. Mineral Processing and Extractive Metallurgy, 2008, 117(3): 137-146.
[15] DENIZ T M, SONER A N H, FIKRET T. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1-4): 169-176.
[16] LECLERC N, MEUX E, LECUIRE J M. Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitrilotriacetate anion and hexahydrated ferric chloride [J]. Journal of Hazardous Materials, 2002, 91(1-3): 257-270.
[17] LI Mi, PENG Bing, CHAI Li-yuan, PENG Ning, XIE Xian-de, YAN Huan. Technological mineralogy and environmental activity of zinc leaching residue from zinc hydrometallurgical process [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1480-1488.
[18] PENG Ning, PENG Bing, CHAI Li-yuan, LI Mi, WANG Ji-ming, YAN Huan, YUAN Yuan. Recovery of iron from zinc calcines by reduction roasting and magnetic separation [J]. Minerals Engineering, 2012, 35: 57-60.
[19] YAN Huan, CHAI Li-yuan, PENG Bing, LI Mi, LIU Wei, PENG Ning, HOU Dong-ke. Reduction roasting of high iron-bearing zinc calcine under a CO-CO2 gas: An investigation of the chemical and mineralogical transformations [J]. JOM, 2013, 65(11): 1589-1596.
[20] WANG Ji-ming. Study on reduction roasting of zinc calcine and selective leaching [D]. Changsha: Central South Unversity, 2012. (in Chinese)
[21] EL-SHOBAKY H G, RADWAN N R E. Investigation of solid-solid interactions in NiO/Fe2O3 system doped with ZnO [J]. Thermochimica Acta, 2003, 398(1, 2): 223-231.
[22] NORDHEI C, MATHISEN K, SAFONOVA O, van BEEK W, NICHOLSON D G. Decomposition of carbon dioxide at 500 °C over reduced iron, cobalt, nickel, and zinc ferrites: A combined XANES-XRD study [J]. The Journal of Physical Chemistry C, 2009, 113(45): 19568-19577.
[23] ZHOU Guo-hua. Technology and theory studies of upgrading silver recovery of flotation from zinc leaching residues [D]. Changsha: Central South Unversity, 2002. (in Chinese)
[24] ZHANG Lin-ye. Stdudy on the mechanism and technology of indium and zinc leaching from zinc leach residues containing indium-bearing zinc ferrite under microwave heating [D]. Nanning: Guangxi University, 2014. (in Chinese)
[25] ZHANG Lin-ye, MO Jia-mei, LI Xuan-hai, PAN Liu-ping, LIANG Xin-yuan, WEI Guang-tao. A kinetic study of indium leaching from indium-bearing zinc ferrite under microwave heating [J]. Metallurgical & Materials Transactions B, 2013, 44(6): 1329-1336.
(Edited by YANG Hua)
Cite this article as: PENG Bing, PENG Ning, LIU Hui, XUE Ke, LIN Dong-hong. Comprehensive recovery of Fe, Zn, Ag and In from high iron-bearing zinc calcine [J]. Journal of Central South University, 2017, 24(5): 1082-1089. DOI: 10.1007/s11771-017-3511-z.
Foundation item: Project(2014FJ1011) supported by the Major Science and Technology Project of Hunan Province, China; Project(51574295) supported by the National Natural Science Foundation of China
Received date: 2016-03-18; Accepted date: 2016-07-21
Corresponding author: LIU Hui, Professor; Tel: +86-731-88830577; E-mail: 45695758 @qq.com