文章编号:1004-0609(2010)09-1849-06
偏钛酸型锂吸附剂的合成及吸附性能
张丽芬, 陈白珍, 石西昌, 马立文, 陈 亚
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:以TiO2和Li2CO3为原料,采用固相法合成偏钛酸型锂吸附剂前躯体Li2TiO3,将该前躯体经过盐酸洗脱锂,得到偏钛酸型锂吸附剂H2TiO3,其中锂的抽出率达到98.86%,钛几乎不溶损。对TiO2、Li2TiO3、H2TiO3以及H2TiO3吸附锂后的样品进行XRD和SEM表征。研究偏钛酸型锂吸附剂H2TiO3对锂离子的吸附性能,并用伪一级动力学方程和伪二级动力学方程对吸附过程进行拟合,计算相应的速率常数。结果表明:H2TiO3对锂离子具有较大的吸附能力,在LiOH溶液中对锂离子的吸附容量为39.8 mg/g;吸附过程符合伪二级动力学方程,表明吸附过程主要为化学吸附,吸附平衡数据符合Langmuir 等温吸附方程。
关键词:锂吸附剂;Li2TiO3;吸附动力学;伪二级动力学
中图分类号:O614.111 文献标志码:A
Synthesis and adsorption property of H2TiO3 type adsorbent
ZHANG Li-fen, CHEN Bai-zhen, SHI Xi-chang, MA Li-wen, CHEN Ya
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: H2TiO3 type adsorbent was obtained by acid-modifying adsorbent precursor Li2TiO3, which was synthesized by a simple solid-phase reaction between Li2CO3 and TiO2. The extraction rate of Li+ from Li2TiO3 is 98.86%,and while almost no Ti4+ is extracted. TiO2, Li2TiO3, H2TiO3 and products of H2TiO3 adsorbing Li+ were characterized by XRD and SEM. The lithium adsorption properties were investigated by adsorption kinetics and adsorption isotherm. The kinetic data were studied by the pseudo-first-order and pseudo-second-order kinetic models. The rate constants of adsorption for these kinetic models were calculated. The results indicate that H2TiO3 type adsorbent shows superior adsorptive capacity of Li+, with the value of 39.8 mg/g in LiOH solution. The adsorption process obeys pseudo-second-order rate equation, and the process can be seen as chemical adsorption. The equilibrium data can be described well by the Langmuir isotherm equation.
Key words:lithium adsorbent;Li2TiO3;adsorption kinetics; pseudo-second-order kinetics
人们对锂产品的需求不断增加,仅靠提取固态锂矿石中的锂已不能满足人类对锂的需求,因此对液态锂的提取具有重要意义。含锂溶液主要为海水、盐湖卤水等,它们的主要特点是含锂量低。传统的沉淀法、溶剂萃取法和盐析法[1-2]等,不适合对含锂量低的海水、盐湖卤水进行锂的提取,而制备适合的离子筛通过吸附法吸附锂是一种有发展前景的方法。对于锂离子筛目前主要集中研究锰系锂离子筛[3-5],但是锰系锂离子筛存在锰溶损大,循环次数少等不足,而钛系锂离子筛具有溶损少、结构稳定等优点。董殿权等[6]应用溶胶-凝胶法合成出Li4Ti5O12,并对其进行酸洗脱锂得到离子筛,锂的抽出率为81.5%,钛离子的抽出率在4.2%以下,对锂离子的吸附容量达到42.30 mg/g;钟辉[7-8]以混晶型二氧化钛(其中锐钛型二氧化钛占83.1%)为原料用固相法合成Li2TiO3,经过酸洗得到锂离子筛,其饱和吸附容量为28.76 mg/g,而且对高钙镁、低锂含量的气田卤水中的锂具有特殊的分离效果。目前,关于偏钛酸型锂吸附剂以及其对Li+吸附过程的动力学和吸附模型的研究比较少。本文作者采用混晶型二氧化钛,用固相法合成Li2TiO3,并对其进行酸浸,洗脱锂离子得到锂吸附剂,利用XRD、SEM对样品的晶体结构和表面形貌进行表征,对锂吸附剂的吸附性能进行研究。
1 实验
1.1 锂吸附剂前躯体 Li2TiO3及锂吸附剂H2TiO3的合成
将TiO2(98%,国药集团化学试剂有限公司)和Li2CO3(97%,天津市光复精细化工研究所)按不同的配比(n(Li2CO3)?n(TiO2)=1.00,1.02,1.05,1.08)、以无水乙醇作为介质将其混合均匀,置于85 ℃烘箱中烘干,在850 ℃下焙烧24 h,得到偏钛酸型锂吸附剂前躯体Li2TiO3。
将锂吸附剂前躯体Li2TiO3放入0.1 mol/L的盐酸中,置于恒温磁力搅拌器中在60 ℃下搅拌,直到Li2TiO3中的锂离子抽出达到平衡,过滤、洗涤、烘干得到偏钛酸型锂吸附剂H2TiO3。
1.2 吸附性能
分别称取一系列的0.5 g锂吸附剂H2TiO3放入带塞子的玻璃瓶中,在瓶中分别加入50 mL 694 mg/L的LiOH 溶液,将玻璃瓶密封置于震荡器中恒温震荡,在不同的时间间隔取样用原子吸收法测定吸附后溶液中锂离子的浓度,运用式(1)计算锂吸附剂对锂离子的吸附容量:
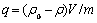 (1)
(1)
式中:q为每克锂吸附剂吸附Li+的量;ρ0为Li+的起始浓度;ρ为不同时间的Li+的浓度;V为溶液的体积;m为吸附剂的质量。
分别称取一系列0.1 g的吸附剂放入带塞子的玻璃瓶中,在瓶中分别加入50 mL不同浓度的LiCl溶液中, pH=10.1(用体积比为1?4的0.1 mol/L NH4Cl和0.1 mol/L NH3·H2O的缓冲溶液调节),将玻璃瓶密封置于震荡器中恒温震荡直到吸附平衡,研究吸附动力学 性质。
1.3 产品的分析与表征
采用X射线衍射仪(Rigaku D?max 2550型,日本生产)对TiO2、Li2TiO3、H2TiO3以及H2TiO3吸附锂后的样品进行X射线衍射分析,工作参数如下:Cu Kα靶(λ=0.154 056 nm),扫描电压40 kV,电流100 mA,步宽0.02?,扫描速度2 (?)/min,扫描范围10?~85?。用扫描电子显微镜(JSM-6360lv型,日本生产)观察Li2TiO3、H2TiO3以及H2TiO3吸附锂后样品的形貌。
采用原子吸收法(TAS-990 F型,北京普析通用仪器有限责任公司生产)测定溶液中锂离子的含量;以二安替比林甲烷作为显色剂用分光光度法(WFJ-7200型,尤尼柯(上海)仪器有限公司生产)测定溶液中钛离子的含量。
2 结果与讨论
2.1 锂吸附剂前躯体 Li2TiO3
图1所示为TiO2和Li2CO3与TiO2按不同配比合成的Li2TiO3的XRD谱。从图1(a)可以看出,TiO2中包含锐钛相(晶格常数:a=b=0.378 52 nm,c=0.951 39 nm(JCPDS 21—1272))和金红石相(晶格常数:a=b=0.459 33 nm,c=0.295 92 nm(JCPDS 21—1276))两种晶相,且金红石相的衍射峰强于锐钛相的。二氧化钛中金红石相TiO2的含量(x)可采用式(2)计算[9]:
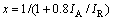 (2)
(2)
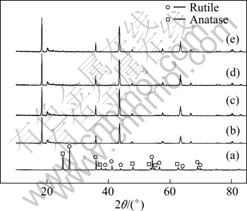
图1 TiO2和Li2CO3与TiO2按不同配比合成的Li2TiO3的XRD谱
Fig.1 XRD patterns of TiO2 and Li2TiO3 synthesized with different molar ratios of Li2CO3 to TiO2: (a) TiO2; (b) n(Li2CO3)?n(TiO2)=1.00;(c) n(Li2CO3)?n(TiO2)=1.02; (d) n(Li2CO3)?n(TiO2)=1.05; (e) n(Li2CO3)?n(TiO2)=1.08
式中:IA为锐钛相TiO2的最强衍射峰的积分强度,出现在2θ=25.24?处;IR为金红石相TiO2的最强衍射峰的积分强度,出现在2θ=27.36?处。根据或(1)计算得到金红石相TiO2的含量为64.19%;锐钛相TiO2的含量为35.81%。
从图1可以看出;采用4种不同配比都可以得到纯相的Li2TiO3;随着锂过量的增大,相应角度的衍射峰强度增加,选择配比n(Li2CO3)?n(TiO2)=1.00时合成得到的Li2TiO3作为锂吸附剂的前躯体。
2.2 锂吸附剂H2TiO3
锂吸附剂前躯体Li2TiO3经过盐酸洗脱锂离子得到锂吸附剂H2TiO3。图2所示为锂的抽出率与抽取时间的变化曲线。由图2可以看出,Li2TiO3经过酸洗1 d的抽出率达到83.64%,之后随时间的延长抽出率缓慢增加,到第4 d锂抽出率达到98.86%,而钛的溶损率小于0.1%。
图3所示为Li2TiO3、H2TiO3以及H2TiO3吸附Li+后的XRD谱,其中曲线(a)所示为纯相的单斜晶相Li2TiO3的XRD谱(晶格常数:a=0.506 9 nm,b=0.879 9 nm,c=0.975 9 nm(JCPDS 33—831));曲线(b)所示为
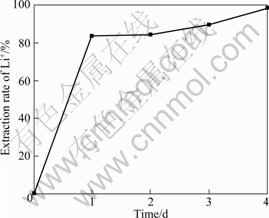
图2 锂的抽出率与抽取时间的变化曲线
Fig.2 Variation of Li+ exchange rate with reaction time
Li2TiO3经过盐酸洗脱Li+后得到的H2TiO3的XRD谱。从图3(b)可以看出衍射峰相对酸洗前向右偏移,强度明显减弱,而且出现衍射峰宽化,可能是Li2TiO3进行酸洗脱锂时,发生了氢离子(半径约为0.001 2 nm)和锂原子(半径约为0.076 nm)的交换,从而造成晶胞缩小。从图3(b)还可以看出部分衍射峰消失,(002)晶面衍射峰最强,可能是经过酸洗后择优取向加强,在c轴方向生长较快。图3中曲线(c)所示为H2TiO3吸附Li+后的XRD谱,可以看出吸附后与吸附前(见曲线(b))的谱相像,说明吸附后结构没有发生明显变化。
图4所示为Li2TiO3、 H2TiO3、H2TiO3吸附Li+后的SEM像。由图4(a)可以看出用固相法合成得到的Li2TiO3颗粒比较均匀,近似圆形,粒径大小为1~2 μm。从图4(b)可以看出,Li2TiO3经过酸洗脱锂得到H2TiO3后颗粒变小,而且出现了不规则的微裂纹,这可能是经过酸洗脱锂时发生氢离子和锂离子的交换,晶胞缩小。从图4(c)可以看出,吸附锂后颗粒比吸附前大,而且出现不规则的多边形以及层状结构,说明在吸附过程中H2TiO3颗粒在某个方向生长较快,造成层状结构明显,此结果与图3(b)、(c)所示的XRD谱分析一致。
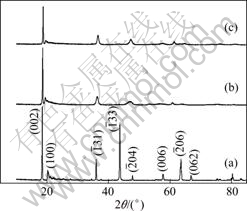
图3 Li2TiO3, H2TiO3以及H2TiO3吸附Li+后的XRD谱
Fig.3 XRD patterns of Li2TiO3 (a), H2TiO3 (b) and products (c) of H2TiO3 after adsorbing Li+

图4 Li2TiO3,H2TiO3以及H2TiO3吸附Li+后的SEM像
Fig.4 SEM images of Li2TiO3(a);H2TiO3 (b) and products (c) of H2TiO3 after adsorbing Li+
2.3 吸附性能
2.3.1 饱和吸附容量
图5所示为锂吸附剂H2TiO3在浓度为694 mg/L的LiOH溶液(曲线(a))和浓度为282.5 mg/L(曲线(b))与87.5 mg/L(曲线(c))的LiCl溶液中吸附容量随时间的变化图。由图5可知,无论是在LiOH溶液还是在LiCl溶液中,吸附容量随时间的延长而不断增加,在第1 d吸附容量增加比较快,随后随时间的延长吸附容量缓慢增大,到第8 d吸附基本达到平衡,吸附容量分别为39.8、30.9、28.63 mg/g。这是因为吸附剂的吸附中心为具有酸式离解能力的—OH基团,吸附剂 S—OH与Li+按等当量交换[10-11]其反应式为
S—OH+ Li+→S—OLi + H+ (3)
因此,吸附剂在中性或碱性溶液中对锂离子具有较强吸附能力。当溶液中锂离子不断被吸附,释放出质子,溶液中的OH-被不断消耗,随着时间的延长吸
附容量缓慢增加,直到吸附平衡。所以,吸附剂在LiOH溶液中的吸附容量高于在LiCl溶液的。
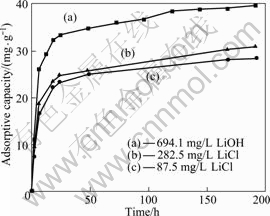
图5 在不同溶液中吸附剂H2TiO3的吸附容量随时间的变化
Fig.5 Variations of adsorptive capacity of H2TiO3 with time in different solutions
2.3.2 吸附动力学
盐湖卤水中的锂一般以LiCl形式存在,因此,对吸附剂在不同浓度的LiCl 溶液中的吸附动力学进行研究。关于吸附剂对金属离子的吸附动力学研究已有不少报道,目前,伪一级动力学式(4)模型和伪二级动力学式(5)模型被广泛应用[12-14]来研究吸附机理,确定吸附过程的速率常数。
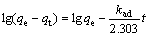 (4)
(4)
 (5)
(5)
式中:qe和qt分别为在吸附平衡和吸附时间为t时的吸附剂对Li+的吸附容量;kad和k分别为伪一级动力学模型和伪二级动力学模型的吸附速率常数。
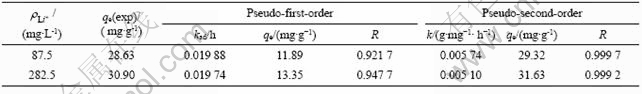
用伪一级动力学方程和伪二级动力学方程对图5中曲线(b)和(c)进行线性拟合,结果如图6和表1所示。由图6和表1可以看出,用伪二级动力学方程拟合的线性相关系数比用伪一级动力学方程拟合的大,达到0.999。所以,可以认为吸附剂在LiCl 溶液中的吸附动力学符合伪二级动力学模型。根据伪二级动力学方程,求出2种浓度下的速率常数分别为0.005 74和0.005 1 g/(mg·h);平衡吸附容量分别为29.32和31.63 mg/g,此吸附容量与图5所示的实验得到的吸附容量28.63和30.9 mg/g相近。这进一步说明吸附剂在LiCl溶液中的吸附符合伪二级动力学方程,表明吸附过程主要为化学吸附[15]。
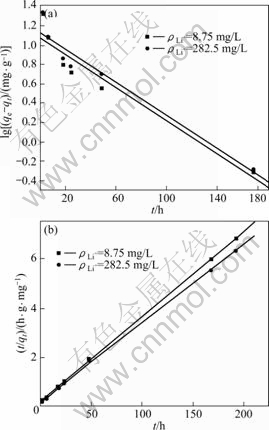
图6 不同Li+ 浓度下吸附剂H2TiO3吸附锂的伪一级动力学和伪二级动力学曲线
Fig.6 Pseudo-first-order (a) and pseudo-second-order (b) kinetic plots for Li+ adsorption of H2TiO3 in solutions with different Li+ concentrations
表1 不同Li+ 浓度下吸附剂H2TiO3吸附Li+的动力学参数
Table 1 Kinetic parameters for Li+ adsorption of H2TiO3 in solutions with different concentrations
2.3.3 吸附等温线
锂吸附剂对Li+的吸附可以通过Langmuir吸附等温方程和Freundlich吸附等温方程拟合[16]:
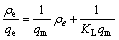 (6)
(6)
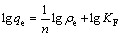 (7)
(7)
式中:ρe 是吸附平衡后溶液中Li+的浓度;qe是平衡吸附容量;qm是最大吸附容量;KL为Langmuir实验常数;n和KF是与吸附容量和吸附速率有关的Freundlich常数。
分别用Langmuir吸附等温方程和Freundlich吸附等温方程对吸附剂在不同浓度的LiCl溶液中平衡时的平衡浓度和吸附容量进行拟合,结果如图7和表2
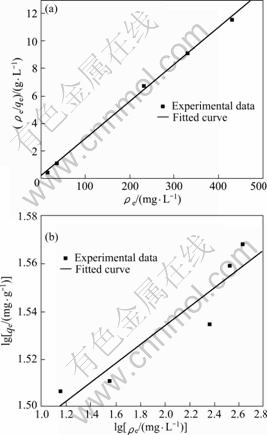
图7 Langmuir和Freundlich等温线
Fig.7 Langmuir(a) and Freundlich(b) isotherms
表2 吸附剂H2TiO3吸附Li+的等温吸附模型及参数
Table 2 Type and parameters of isothermal adsorption for Li+ adsorption of H2TiO3

所示。从图7和表2 可以看出:用Langmuir吸附等温方程进行拟合的线性关系比Freundlich拟合的好,线性相关系数达到0.998 4。说明吸附剂对锂离子的吸附为单层吸附,最大吸附容量为37.01 mg/g。
3 结论
1) 以TiO2 (金红石相TiO2含量为64.19%;锐钛相TiO2的含量为35.81%)与Li2TiO3为原料,采用固相法可以合成单一晶相Li2TiO3,经过盐酸洗脱Li+,可以得到锂吸附剂H2TiO3,其中锂的抽出率达到98.86%,钛的溶损小于0.1%。
2) 吸附实验证明锂吸附剂H2TiO3对锂离子具有较大的吸附能力,在694 mg/L的LiOH溶液中的平衡吸附容量达到39.8 mg/g。
3) 通过对吸附动力学的研究得出锂吸附剂H2TiO3对LiCl溶液中Li+的吸附过程符合伪二级动力学方程,表明吸附过程主要为化学吸附;在87.5 mg/L和282.5 mg/L的LiCl溶液中平衡吸附容量分别为29.32和31.63 mg/g;吸附速率常数分别为 0.005 74和0.005 10 g/(mg· h)。
4) 通过对吸附等温方程的研究得出锂吸附剂H2TiO3对LiCl溶液中Li+的吸附过程符合Langmuir等温吸附,表明吸附过程为单层吸附,最大吸附容量为37.01 mg/g。
REFERENCES
[1] 陈 婷, 闫书一, 康自华. 我国盐湖卤水提锂的研究进展[J]. 盐业与化工, 2006, 36(2): 19-21.
CHEN Ting, YAN Shu-yi, KANG Zi-hua. Progress on the extraction of lithium from the salt lake brine in china[J]. Salt and Chemical Industry, 2006, 36(2): 19-21.
[2] 黄维农, 孙之南, 王学魁, 乜 贞, 卜令忠. 盐湖提锂研究和工业化进展[J]. 现代化工, 2008, 28(2): 14-19.
HUANG Wei-nong, SUN Zhi-nan, WANG Xue-kui, NIE Zhen, BU Ling-zhong. Progress in industrialization for lithium extraction from salt lake[J]. Modern Chemical Industry, 2008, 28(2): 14-19.
[3] WANG Lu, MENG Chang-gong, MA Wei. Study on Li+ uptake by lithium ion-sieve via the pH technique[J]. Colloids and Surface A: Physicochemical and Engineering Aspects, 2009, 334(1): 34-39.
[4] ZHANG Qin-hui, SUN Shu-ying, LI Shao-peng, JIANG Hao, YU Jian-guo. Adsorption of lithium ions on novel nanocrystal MnO2[J]. Chemical Engineering Science, 2007, 62(18): 4869-4874.
[5] WANG Lu, MA Wei, LIU Ru, LI Hai-yan, MENG Chang-gong. Correlation between Li+ adsorption capacity and the preparation conditions of spinel lithium manganese precursor[J]. Solid State Ionics, 2006, 177(17): 1421-1428.
[6] 董殿权, 张凤宝, 张国亮, 刘亦凡. Li4Ti5O12的合成及对Li+的离子交换动力学[J]. 物理化学学报, 2007, 23(6): 950-954.
DONG Dian-quan, ZHANG Feng-bao, ZHANG Guo-liang, LIU Yi-fan. Synthesis of Li4Ti5O12 and its exchange kinetics with Li+[J]. Acta Physico-Chimica Sinica, 2007, 23(6): 950-954.
[7] 钟 辉. 偏钛酸型锂交换体制备、机理及其从液态锂矿提锂研究[D]. 成都: 成都理工大学材料与化学化工学院, 2004: 53-54.
ZHONG Hui. Research on the preparation and mechanism of the H2TiO3-type lithium exchanger and its application to the extraction of Li+ from the brine[D]. Chengdu: College of Materials and Chemistry and Chemical Engineering, Chengdu University of Technology, 2004: 53-54.
[8] 钟 辉. 偏钛酸型锂离子交换剂的交换性质及从气田卤水中提锂[J]. 应用化学, 2000, 17(3): 307-309.
ZHONG Hui. Property of H2TiO3 type ion-exchangers and extraction of lithium from brine of natural gas wells[J]. Applied Chemistry, 2000, 17(3): 307-309.
[9] ZHANG Qing-hong, GAO Lian, GUO Jing-kun. Effects of calcination on the photocatalytic properties of namosized Tio2 powers prepared by TiCl4 hydrolysis[L]. Applied Catalysis B: Environmental, 2000, 26: 207-215.
[10] 闫树旺, 钟 辉, 周永兴. 二氧化钛吸附剂的研制及从卤水中提锂[J]. 离子交换与吸附, 1992, 8(3): 222-228.
YAN Shu-wang, ZHONG Hui, ZHOU Yong-xing. Study on ionic sieve of titanium oxide and lithium recovery from brines[J]. Ion Exchange and Adsorption, 1992, 8(3): 222-228.
[11] 钟 辉, 殷辉安. 偏钛酸型离子交换剂表面性质与选择吸附性研究[J]. 离子交换与吸附, 2003, 19(1): 55-60.
ZHONG Hui, YIN Hui-an. Study on the properties of the surface and absorb of Li+ ion-exchange of H2TiO3 type[J]. Ion Exchange and Adsorption, 2003, 19(1): 55-60.
[12] ZHOU Li-min, WANG Yi-ping, LIU Zhi-rong, HUANG Qun-wu. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modi?ed magnetic chitosan microspheres[J]. Hazardous Materials, 2009, 161(2): 995-1002.
[13] BENHAMMOU A, YAACOUBI A, NIBOU L, TANOUTI B. Adsorption of metal ions onto Moroccan stevensite: Kinetic and isotherm studies[J]. Colloid and Interface Science, 2005, 282(2): 320-326.
[14] BARKAT M, NIBOU D, CHEGROUCHE S, MELLAH A. Kinetics and thermodynamics studies of chromium(VI) ions adsorption onto activated carbon from aqueous solutions[J]. Chemical Engineering and Processing, 2009, 48(1): 38-47.
[15] NAIYA T K, BHATTACHARYA A K, DAS S K. Removal of Cd(II) from aqueous solutions using clari?ed sludge[J]. Colloid and Interface Science, 2008, 325(1): 48-56.
[16] WANG Lu, MENG Chang-gong, HAN Mei, MA Wei. Lithium uptake in fixed-pH solution by ion sieves[J]. Colloid and Interface Science, 2008, 325(1): 31-40.
(编辑 杨 华)
基金项目:国家科技支撑计划“十一五”重大项目(2008BAB35B04)
收稿日期:2009-10-16;修订日期:2009-12-08
通信作者:石西昌,副教授,博士;电话:0731-88877352;E-mail:xichang.shi@gmail.com