


Trans. Nonferrous Met. Soc. China 22(2012) 2021-2026
Thermal stability and Judd-Ofelt analysis of optical properties of Er3+-doped tellurite glasses
REN Fang, MEI Yu-zhao, GAO Chao, ZHU Li-gang, LU An-xian
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 6 September 2011; accepted 10 January 2012
Abstract: Er3+-doped TeO2-ZnO-Na2O-B2O3-GeO2 (TZNBG) glasses were prepared by melt-quenching method. Differential scanning calorimetry (DSC) and thermal mechanical analysis (TMA) were used to calculate thermal parameters: crystallization temperature (Tx), glass transition temperature (Tg) and thermal expansion (α). Besides, Judd-Ofelt theory is applied to analyzing absorption spectra. Intensity parameters Wλ (λ=2, 4, 6), transition probabilities Aed, radiative lifetime τi, and branching ratios β of Er3+ transitions were obtained. Emission cross-section σemis of 4I13/2→4I15/2 transition of Er3+ was calculated according to the theory of McCumber. All of the parameters indicate that the thermal stability and optical properties of Er3+-doped TZNBG glasses are improved effectively.
Key words: tellurite glasses; thermal stability; Judd-Ofelt theory; spectroscopic properties
1 Introduction
Er3+-doped tellurite glasses possess large emission cross-section, flattened broad bandwidth, excellent transmission in visible and near IR, relatively low phonon energy and a large refractive index compared with other oxide glasses [1,2]. Due to their excellent properties, the Er3+-doped tellurite glasses are used as candidates for broad band amplifiers [3,4]. But, serious drawback for tellurite glasses is their relatively low thermal stability. These disadvantages result in that ?bers made from these glasses are too fragile, so they do not show light guiding at all [5]. Furthermore, the tellurite glasses are hard to applicate due to their drawbacks. And so far there are many studies about improving their thermal stability. JLASSI et al [6] reported that both thermal stability and quantum efficiency were improved by adding P2O5 to tellurite glasses. EL-MALLAWANY et al [7] made quantitative analysis of thermal properties of tellurite glass with the structure parameters like the average cross-link density, the number of bonds per unit volume, and the average stretching force constant, but the optical properties were not reported. It is necessary to possess excellent thermal stability for the tellurite glasses for further application in optical fiber amplifiers.
In this work, improving thermal stability and optical properties is the main purpose. The Er3+-doped TeO2-ZnO-Na2O-B2O3-GeO2 (TZNBG) glasses were elaborated by melt-quenching method. Both B2O3 and GeO2 introduced into tellurite glass compositions at the same time have been rarely reported. And differential scanning calorimetry (DSC), thermal mechanical analysis (TMA), absorption and emission measurements were performed. In addition, theories of Judd-Ofelt and McCumber were applied to analyzing the optical properties.
2 Experimental
Tellurite glasses with compositions listed in Table 1 were prepared. Er2O3 (99.95%, mass fraction) was added into all glass samples. Na2O and B2O3 were introduced in the form of Na2CO3 and H3BO3, respectively. Batches of 15 g were prepared from commercial powders of TeO2 (99.999%), ZnO (99.9%), Na2CO3 (99.9%), H3BO3 (99.9%) and GeO2 (99.99%). The powders were mixed in a mortar and immersed in CCl4 which was as a reagent of dehydration for 10 min at room temperature. The homogeneous mixture was melted in an alumina crucible at 600 ℃ for 0.5-1 h, then at 900 ℃ for 1-2 h in a furnace. When the melting was completed, the liquids were poured into preheated massive graphite plates at 300-320 ℃ and annealed at this temperature for 3 h. The glasses were cooled to 100 ℃ after 24 h. The glass blocks prepared were cut into desired dimensions and optically polished for different measurements.
Table 1 Compositions of Er3+-doped tellurite glasses

Thermal analysis was performed with a TAS100 thermal analytical instrument and Netzsch DTA 449 PC differential scanning calorimeter at a heating rate of 10 ℃/min. The absorption spectra were recorded by a Perkin-Elmer Lambda-900 spectrophotometer in the wavelength range of 400-1700 nm. And emission spectra were collected by using Edinburgh Instruments Ltd FLSP 920 spectrophotometer, with 976 nm laser diode as the excitation source. Glass samples for optical and spectroscopic measurements were cut and polished in the demensions of 15 mm×15 mm×3 mm and all the optical measurements were carried out at room temperature.
3 Results and discussion
3.1 Thermal stability
In order to evaluate the thermal stability of glass samples, measurements of DSC and TMA were performed. Figure 1 presents the DSC curve of TZNBG glasses. The DSC curve shows glass transition temperature (Tg) and crystallization temperature (Tx) in glass sample. And thermal expansion coefficient α was obtained by TMA. Results of thermal parameters are listed in Table 2. ΔT is identified as: ΔT=Tx-Tg. And the ΔT has been frequently used as a rough measure of the glass thermal stability [8]. Since ?ber fabricating is a reheating process, any crystallization during the process will lead to more scattering loss of the ?ber and then damage the optical properties. To achieve a large range of working temperature during the ?ber fabricating and to obtain glass fiber with superior optical properties, it is desired that ΔT of glass as large as possible [9]. The Tg and glass softening temperature (Tf) increase for the B2O3 and GeO2 introduced into glass compositions. And the crystallization peaks were extremely weak in samples of TZNBG glass. Furthermore, the value of ΔT is larger than 134 ℃. In addition, α of TZNBG glass samples is in the range of (10.938-12.279)×10-6℃-1. It is much lower than 15.466×10-6℃-1 of TZN glass sample. So, it suggests that introducing B2O3 and GeO2 into tellurite glasses can improve their thermal stability. The reason for the performance change is structure of glass changed after introducing B2O3 and GeO2 into the tellurite glasses [4].
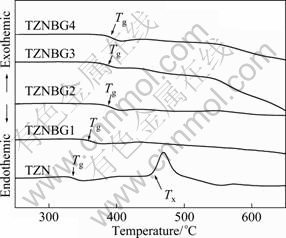
Fig. 1 DSC curves of glass sample
Table 2 Thermal parameters of different glass samples

3.2 Absorption spectra and Judd-Ofelt analysis
The TZNBG 2 glass sample was selected for further optical studies due to its relatively good thermal stability,and the TZN sample was as comparison.
Figure 2 illustrates the absorption spectra of Er3+- doped TZNBG 2 and TZN glass samples. The absorption spectra consist of eight absorption bands at 1531, 978, 796, 652, 544, 521, 488 and 451 nm, corresponding to the absorption from the ground state 4I15/2 to the excited sates of 4I13/2, 4I11/2, 4I9/2, 4F9/2, 4S3/2, 2H11/2, 4F7/2 and 4F5/2 of Er3+, respectively. As we can see the peak position of each transition remains unchanged with adding the B2O3 and GeO2.
The theory of Judd-Ofelt [11,12] is often used to calculate the spectroscopic parameters such as oscillator coefficient ?, intensity parameters Wl (l=2, 4, 6), transition probability Аed, branching ratio β, radiative lifetime τi. The oscillator coefficient for each band is computed by the following expression:
 (1)
(1)
where N is the number of rare earth ions per unit volume; ε(σ) is the molar absorptivity in L/(mol·cm) of band at a mean energy σ in cm-1, which is computed from the measured absorbance for known concentrations N0 of the Er in the glass [13].

Fig. 2 Absorption spectra of Er3+-doped tellurite glasses
According to the Judd-Ofelt theory the oscillator strength of an electric dipole transition (S, L, J→S′, L′, J′) is determined from formula (2):
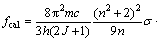
 (2)
(2)
where h is the Planck’s constant; c is the speed of light; m is the mass of electron; n is the refractive index; U(λ) is the doubly reduced unit tensor operator that is taken from Ref. [13]. The Judd-Ofelt parameters Ωλ (λ=2, 4, 6) are obtained by a least-square method and the oscillator strength ? for any transition is evaluated from formula (2). The quality of the fitting of the theoretical oscillator strength values to the measured ones can be expressed by the root-mean-square δrms, which is calculated by:
is the doubly reduced unit tensor operator that is taken from Ref. [13]. The Judd-Ofelt parameters Ωλ (λ=2, 4, 6) are obtained by a least-square method and the oscillator strength ? for any transition is evaluated from formula (2). The quality of the fitting of the theoretical oscillator strength values to the measured ones can be expressed by the root-mean-square δrms, which is calculated by:
 (3)
(3)
where Nbands regards the number of transition bands analyzed.
The values of Ωλ (λ=2, 4, 6) can be applied to calculating the radiative transition probabilities Aed (J′→J′′), for excited levels of rare earth ions from an initial state J′ to a final ground state J′′, is given by the following formula:

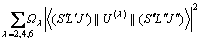 (4)
(4)
The total transition probability (AT) has been evaluated from AT=∑Aed. The branching ratio can be identified as β=Aed/AT. The radiative lifetime τi was calculated from τi=1/AT. The oscillator strength ? and the intensity parameter (Ωλ) are set out in Table 3, and radiative transition probability (Aed), branching ration (β) and the radiative lifetime (τi) are listed in Table 4.
Table 3 Measured (fmea) and calculated (fcal) oscillator coefficient and intensity parameter (Ωλ) for Er3+- doped tellurite glasses by Judd-Ofelt theory

According to the theory of JACOBS and WEBER [14], erbium emission intensity can be characterized by W4 and W6 parameters. The smaller value of W4/W6 corresponds to higher intensity of laser transition 4I13/2→4I15/2 of Er3+ [15]. In the present work, the value of W4/W6 is estimated to be 0.783 for Er3+ in TZNBG 2 glass sample, which indicates that the transition 4I13/2→4I15/2 is more efficient than that in other glass samples [6,10,10]. The values of W4/W6 of different glass samples are included in Table 5.
3.3 Emission spectra and emission cross-section
Figure 3 exhibits the emission spectra for the 4I13/2→4I15/2 transition of Er3+-doped TZN and TZNBG 2 glass samples. According to the McCumber theory [17], the emission cross-section σemis can be determined by 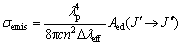 (5)
(5)
where λp is the peak fluorescence wavelength, and ?λeff is the effective line-width of emission band, which can be calculated using the following equation:
 (6)
(6)
where Imax is the maximum intensity at the fluorescence emission peak.
Table 4 Radiative transition probability (Aed), branching ratio (β) and radiative lifetime (τi) of energy levels of Er3+-doped in tellurite glasses

Table 5 Value of W4/W6 of different glass samples


Fig. 3 Emission spectra of 4I13/2→4I15/2 transition of Er3+ in all glasses under 976 nm excitation
Table 6 gives the emission cross-section σemis, and the full width at half maxima (FWHM) of the emission peak of 4I13/2→4I15/2 transition of Er3+ in different glass matrices. Bandwidth properties of the optical amplifier can be evaluated from the product of σemis and FWHM, and the larger the better. The value of σemisτi can be applied to evaluating the gain of bandwidth [16]. As the results present in Table 6, these parameters of TZNBG 2 glass sample are more excellent than those of other glass sample. Further more, the value of σemis×FWHM of tellurite glass samples that introduced B2O3 and GeO2 is larger than TZN glass samples too [7,17]. On the other hand, the product of σemis and τi is the largest among these glass samples. So, the tellurite glass samples contain B2O3 and GeO2 are more suitable to be used as candidate for broad band optical amplifiers.
Table 6 Emission cross-section σemis, FWHM and radiative lifetime τi of 4I13/2→4I15/2 transition of Er3+ in different glass samples

4 Conclusions
1) The results of DSC and thermal mechanical analysis reveal that Er3+-doped TZNBG glasses possess high thermal stability, the ΔT is higher than 134 ℃ and the α is in the range of (10.938-12.279)×10-6℃-1.
2) The value of W4/W6 is estimated to be 0.783 for Er3+ in TZNBG glass sample, which indicates that the transition 4I13/2→4I15/2 is more efficient than that in other glass samples. The emission cross-section σemis, and FWHM of 1530 nm emission peak of 4I13/2→4I15/2 transition of the Er3+ suggest that the TZNBG glass samples have good bandwidth properties and high gain of bandwidth.
3) On the basis of all the data obtained, the thermal stability and optical properties of Er3+-doped TZNBG glasses are improved.
References
[1] WANG J S, VOGEL E M, SNITZER E. Tellurite glass: A new candidate for fiber devices [J]. Optical Materials, 1994, 3: 187-203.
[2] WEBER M J, MYERS J D, BLACKBURN D H. Optical properties of Nd3 + in tellurite and phosphotellurite glasses [J]. Journal of Applied Physics, 1981, 52: 2944-2949.
[3] BERNESCHI S, NUNZI CONTI G, B?NY?SZ I, KHANH N Q, FRIED M, P?SZTI F, BRENCI M, PELLI S, RIGHINI G C. Ion beam irradiated channel waveguides in Er3+-doped tellurite glass [J]. Applied Physics Letters, 2007, 90: 121136.
[4] XIANG Shen, NIE Qiu-hua, XU Tie-feng, DAI Shi-xun, WANG Xun-si. Effect of B2O3 on luminescence of erbium doped tellurite glasses [J]. Spectrochimica Acta Part A, 2007, 66: 389-393.
[5] RIVERA V A G, RODRIGUEZ E, CHILLCCE E F, CESAR C L, BARBOSA L C. Waveguide produced by ?ber on glass method using Er3+-doped tellurite glass [J]. Journal of Non-Crystalline Solids, 2007, 353: 339-343.
[6] JLASSI I, ELHOUICHET H, FARTHOU M. Judd-Ofelt analysis and improvement of thermal and optical properties tellurite glasses by adding P2O5 [J]. Journal of Luminescence, 2010, 130: 2394-2401.
[7] EL-MALLAWANY R, ABBAS AHMED I. Thermal properties of multicomponent tellurite glass [J]. Journal of Materials Science, 2008, 43: 5131-5138.
[8] HRUBY A. Evaluation of glass-forming tendency by means of DTA [J]. Czech Journal of Physics B, 1972, 22: 1187-1193.
[9] DREXHAGE M G, EI BAYOUMI O H, MOYNIYAN C T. Preparation and properties of heavy-metal fluoride glasses containing ytterbium or lutetium [J]. Journal of the American Ceramic Society C, 1982, 65: 168-171.
[10] JOSHI P, SHEN Shao-xiong, JHA A. Er3+-doped boro-tellurite glass for optical ampli?cation in the 1530-1580 nm [J]. Journal of Applied Physics, 2008, 103: 083543.
[11] JUDD B R. Optical absorption intensities of rare-earth ions [J]. Physical Review, 1962, 127: 750-761.
[12] OFELT G S. Intensities of crystal spectra of rare-earth ions [J]. Journal of Chemical Physics, 1962, 37: 511-520.
[13] CARNALL W T, P R F, RAJNAK K. Electronic energy levels in the trivalent lanthanide aquo ions: I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, HO3+, Er3+and Tm3+ [J]. Journal of Chemical Physics, 1968. 49(10): 4424-4442.
[14] JACOBS R R, WEBER M J. Dependence of the 4F3/2→4I11/2 induced-emission cross section for Nd3+ on glass composition [J]. IEEE Journal of Quantum Electron, 1976, 12: 102-111.
[15] YU Chun-lei, HE Dong-bing, WANG Guo-nian, ZHANG Jun-jie, HU Li-li. In?uence of cationic ?eld strength of modi?ers on the 1.53 μm spectroscopic properties of Er3+-doped tellurite glasses [J]. Journal of Non-Crystalline Solids, 2009, 355: 2250-2253.
[16] BILIR G, OZEN G, TATAR D, ?VE?O?LU M L. Judd-Ofelt analysis and near infrared emission properties of the Er3+ ions in tellurite glasses containing WO3 and CdO [J]. Optics Communications, 2011, 284: 863-868.
[17] McCUMBER D E. Theory of phonon-terminated optical masers [J]. Physical Review A, 1964, 134: 299-306.
[18] QIAN Q, WANG Y, ZHANG Q Y, YANG G F, YANG Z M, JIANG Z H. Spectroscopic properties of Er3+-doped Na2O–Sb2O3–B2O3– SiO2 glasses [J]. Journal of Non-Crystalline Solids, 2008, 354: 1981-1985.
掺铒碲酸盐的热稳定性和Judd-Ofelt理论分析
任 芳,梅宇钊,高 超,朱立刚,卢安贤
中南大学 材料科学与工程学院,长沙 410083
摘 要:利用熔融法制备掺铒TeO2-ZnO-Na2O-B2O3-GeO2碲酸盐玻璃。采用差热扫描分析法(DSC)和热分析(TMA)得到玻璃的玻璃转化温度(Tg)、玻璃析晶温度(Tx)、玻璃软化温度(Tf)和热膨胀系数(α),应用Judd-Ofelt理论计算玻璃中Er3+的振子强度Wλ (λ=2, 4, 6),跃迁几率Aed,荧光分支比β,辐射寿命τi。根据McCumber理论计算Er3+离子4I13/2→4I15/2的受激发射截面σemis和荧光半高宽FWHM。得出此体系的玻璃具有高热稳定性和低热膨胀性,具有较高的Er3+离子4I13/2→4I15/2 能级跃迁效率和较好的增益带宽性能。
关键词:碲酸盐玻璃;热稳定性;Judd-Ofelt理论;光谱性能
(Edited by LI Xiang-qun)
Corresponding author: LU An-xian; Tel: +86-731-88877057; E-mail: Luanxian@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)61423-4