原硅酸钙在铝酸钠溶液中的反应行为
李小斌,张 建,刘桂华,陈 滨,齐天贵
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:以CaO和SiO2为原料合成2CaO·SiO2,通过测定SiO2浓度分析原硅酸钙反应活性的变化规律;基于质量守恒,计算渣中钠硅渣和钙硅渣分配比例。实验结果表明,在铝酸钠溶液体系中,反应时间的延长、氧化铝浓度的升高均有利于原硅酸钙的分解和溶出液中二氧化硅浓度的升高,溶出液中二氧化硅浓度最高增幅可分别达到9.97倍和11倍;同时,温度升高会显著促进原硅酸钙的分解与钙硅渣的生成,在136 ℃反应1 h后二氧化硅总反应率可达43.06%;在铝酸钠溶液中加入碳酸钠,可能因“协同效应”而促进原硅酸钙的分解;铝酸钠溶液苛性比变化对原硅酸钙的分解影响不明显。随着纯铝酸钠、碳酸钠和氢氧化钠溶液浓度的升高,原硅酸钙的Zeta电位绝对值增大,其原因可能是溶剂化层相应的Al(OH)4-,CO32-和OH-含量增多,有利于原硅酸钙的分解。
关键词:原硅酸钙;铝酸钠溶液;钠硅渣;钙硅渣;反应行为
中图分类号:TF821 文献标识码:A 文章编号:1672-7207(2009)02-0275-07
Reactive behaviors of calcium silicate in aluminate solutions
LI Xiao-bin, ZHANG Jian, LIU Gui-hua, CHEN Bin, QI Tian-gui
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Reactive behaviors of 2CaO·SiO2 synthesized from CaO and SiO2 was studied in term of concentration of silica, and the proportion of sodium aluminosilicate hydrate and hydrogarnet was also calculated on the basis of conservation of mass. The results show that the prolongation of time or the increase of Al2O3 concentration favors the decomposition of 2CaO·SiO2 and leads to more silica left in aluminate solution. The maximal SiO2 concentration in solution increases by 9.97 and 11 times compared with the minimum SiO2 concentration respectively. The increase of the temperature fairly benefits the decomposition of 2CaO·SiO2 and formation of more hydrogarnet. The total reaction rate of silica reaches 43.06% at 136 ℃ after reaction for 1 h. When Na2CO3 is added into NaAl(OH)4 solution, more 2CaO·SiO2 is decomposed owing to synergism. αK affects slightly the decomposition of 2CaO·SiO2. The absolute value of Zeta potential of 2CaO·SiO2 rises with the increase of pure NaOH, NaAl(OH)4 and Na2CO3 concentration, which means more OH-, Al(OH)4- and CO32- stays in the solvent layer of 2CaO·SiO2 and favors the decomposition of 2CaO·SiO2.
Key words: calcium silicate; aluminate solutions; sodium aluminosilicate bydrate; hydrogamet; reactive behavior
烧结法适用于处理我国占绝大多数的中低品位铝土矿。在熟料烧结过程中,铝转化成易溶液于水的铝酸钠,硅转化成相对稳定、不溶于水的原硅酸钙(β-2CaO·SiO2),从而在熟料溶出过程中实现铝硅分离。但在实际生产中,原硅酸钙在溶出过程中会发生反应,称为二次反应;二次反应后,硅所生成的钠硅渣(Na2O·Al2O3·1.7SiO2·2H2O)和钙硅渣(水化石榴石、3CaO·Al2O3·xSiO2·(6-2x)H2O)进入赤泥,导致碱和氧化铝损失,称为二次反应损失[1-4]。二次反应损失是以熟料的标准溶出率和净溶出率进行计算的,不考虑进入粗液中的硅,其计算的一个依据是:基于烧结法生产氧化铝全流程,粗液中的硅经脱硅后生成的钠硅渣和钙硅渣全部返回生料浆配料系统,回收其中碱和氧化铝。但在讨论原硅酸钙的反应活性(或稳定性)时,有时用二次反应损失来表征,从而容易产生误解。因为原硅酸钙的反应活性实际来自两方面:二次反应损失的一部分硅和进入粗液中的硅,其中,进入粗液中的硅不仅使脱硅系统复杂化,而且使生料浆的配制过程复杂化,降低熟料的铝硅比,降低大窑的产量。因此,需用更精确的概念来表征原硅酸钙的反应活性。
同时,目前人们对二次反应机理也存在争论[5],如: NaOH或Na2CO3分解原硅酸钙引起二次反应[6]; NaAl(OH)4分解原硅酸钙引起二次反应[7-8]。为此,Liu等[9-13]基于热力学计算和纯碱液(氢氧化钠或碳酸钠)中的实验研究,认为硅酸钙在苛性碱溶液中较稳定,在碳酸钠溶液中不稳定,在相同反应条件下,CaO·SiO2比2CaO·SiO2稳定。但在实际生产中,熟料溶出用的调整液主要成分是铝酸钠,并含有10~30 g/L碳酸钠(以Na2O计),而目前人们没有对原硅酸钙在铝酸钠溶液中的反应行为进行系统、全面的研究,不能明确铝酸根离子对原硅酸钙分解反应的作用规律,也不清楚铝酸根离子大量存在条件下,游离碱、碳酸钠等作用规律。
本文作者基于溶液中SiO2浓度变化及渣中钠硅渣和钙硅渣含量的变化结合Zeta电位测定,研究时间、温度、游离苛性碱浓度、碳酸钠浓度、氧化铝浓度等对原硅酸钙与铝酸钠反应行为的影响,阐述原硅酸钙二次反应规律[13]。
1 实 验
1.1 原硅酸钙的制备
将分析纯Ca(OH)2置于马弗炉中于850 ℃灼烧2.5 h得CaO;将分析纯SiO2在110 ℃烘2 h。CaO与SiO2按照摩尔比2?1混合均匀,然后,放入刚玉坩埚中,置于850 ℃马弗炉(长沙中华电炉厂,型号为KSY-120)中预烧10 min后,转入1 300 ℃马弗炉中灼烧2 h,取出、冷却、装入磨口瓶。用D500(siemens)X射线衍射仪对合成的原硅酸钙进行物相分析(图1(a)),主要物相为β, γ-2CaO·SiO2。而铝酸钠溶液与原硅酸钙反应后渣相的物相分析结果如图1(b)所示。结果表明,主要物相是原硅酸钙、钙硅渣(水化石榴石)和钠硅渣。
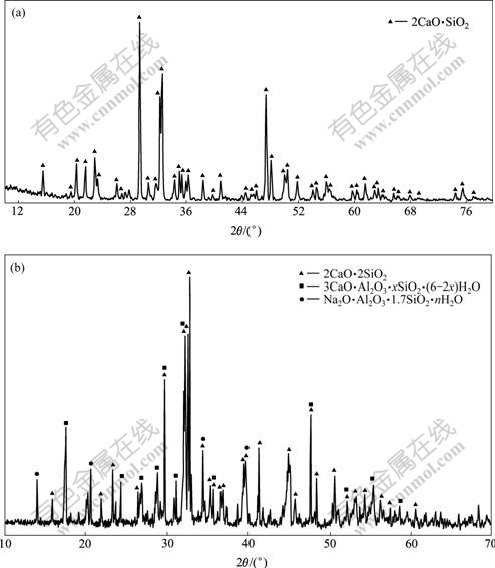
(a) 原硅酸钙;(b) 硅酸钙与铝酸钠溶液反应后
图1 原硅酸钙的反应前后固相XRD谱(100 ℃,60 min)
Fig.1 XRD pattern of 2CaO·SiO2(a) and sediments after reaction between 2CaO·SiO2 and aluminate solution(b)
1.2 硅酸钙的浸出
用化学纯氢氧化铝、氢氧化钠配制一定浓度铝酸钠溶液。每次实验时,在反应器中加入6 g原硅酸钙和100 mL铝酸钠溶液,反应一定时间后取出、过滤,用开水充分洗涤。所得滤液用分光光度法[7]分析SiO2浓度,用容量法测定[7]分析氧化铝和氧化钠浓度;而对得到的渣进行化学成分或物相分析。
1.3 原硅酸钙与不同碱液反应表面Zeta电位的测定方法
称取一定质量的待测原硅酸钙放入预先配制的碱液中,充分分散,得到带有悬浮颗粒的上清液样品;用PHS-3C型pH计(长沙索拓科学仪器设备有限公司生产)测样品的pH值;用JS94H型微电泳仪(上海中晨数字技术设备有限公司生产)测定硅酸钙颗粒表面Zeta电位。
2 结果与讨论
2.1 数据处理
由物相分析结果可知,原硅酸钙在溶出过程中,损失的氧化铝主要存在于钠硅渣和钙硅渣中。溶出后的渣是由钙硅渣、钠硅渣以及没有参与反应的原硅酸钙组成。假设溶液中损失的氧化钠全部进入钠硅渣 中,进而进行计算得到钠硅渣含量及消耗的部分氧化铝,剩余氧化铝全部进入钙硅渣,从而计算得到钙硅渣质量。
渣中钠硅渣的生成量为:
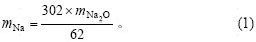
钙硅渣中氧化铝质量为:
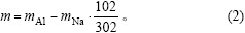
渣中原硅酸钙质量为:
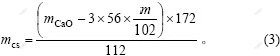
渣中钙硅渣的生成质量为:
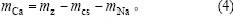
式中:mz为渣的质量;mAl为渣中氧化铝质量;mNa2O为氧化钠质量;mCaO为氧化钙质量。
2.2 原硅酸钙在铝酸钠溶液的反应行为
2.2.1 时间和温度对原硅酸钙与铝酸钠溶液反应的 影响
当原硅酸钙与铝酸钠溶液反应时,时间和温度对反应的影响如图2所示。
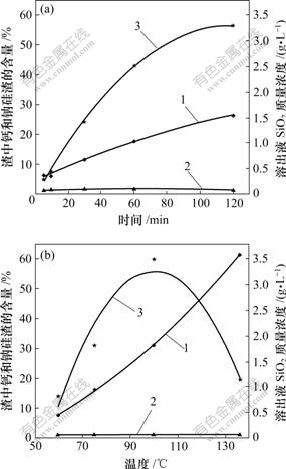
1—钙硅渣;2—钠硅渣;3—SiO2质量浓度
铝酸钠溶液其他成分:Al2O3 121.25 g/L; Na2Ok 127.51 g/L; Na2Oc 21.46 g/L; αk 1.73
(a) 时间对反应的影响(实验条件:温度80 ℃;熟料添加量60 g/L);(b) 温度对反应的影响(实验条件:时间 60 min;熟料添加量60 g/L)
图2 溶出后渣中钙硅渣、钠硅渣生成和溶出液中二氧化硅质量浓度随时间和温度的变化
Fig.2 Influence of leaching time and temperature on SiO2 concentration and distribution of hydrogarnet and sodium aluminosilicate hydrate
从图2(a)可知,随着时间的延长,溶出液中SiO2浓度升高。溶出液中SiO2质量浓度从5 min的0.30 g/L升高到120 min的3.29 g/L,最高增幅可达9.97倍。其主要原因是,在铝酸钠溶液中,在一定温度下,硅
易与铝形成稳定性较强的铝硅酸根离子,从而使得溶液中二氧化硅浓度保持在较高值;同时,渣中钠硅渣质量随反应时间的延长变化不明显,而钙硅渣质量随时间的延长而明显增加。
从图2(b)可知,在温度100 ℃以下,随着温度的提高,溶出液中SiO2质量浓度随之明显增大。但当温度升高到100 ℃以上时,SiO2质量浓度又大幅度下降。这是因为,一方面,温度的提高有利于加快原硅酸钙与铝酸钠溶液之间的反应速度,导致硅酸钙分解程度变大;另一方面,温度过高,有利于提高如下脱硅反应的速度。
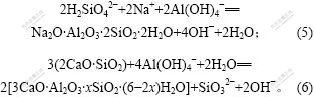
图2(b)所示实验结果还表明:随着温度的升高,钙硅渣量明显增加,而钠硅渣仍变化不明显。渣中钙、钠硅渣生成质量分别为5.68 g和0.14 g,其总含量最高可达62.73%,钙硅渣生成质量最高可为钠硅渣生成质量的40.57倍。这说明温度的升高促进了原硅酸钙与铝酸钠的反应,同时,钙硅渣是二次反应损失的主要形式。
由于二次反应损失是指熟料溶出过程中氧化铝标准溶出率和净溶出率的差值,难以精确反映熟料溶出过程中原硅酸钙的稳定性,因此,本文作者在熟料溶出过程中,将二次反应损失的硅和粗液中的硅相加,提出 “二氧化硅总反应率”概念,重新处理图2中的数据,结果如表1所示。
表1 时间和温度对SiO2反应率的影响
Table 1 Influence of leaching time and temperature on reaction rate of SiO2
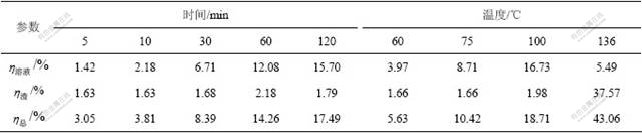
从表1可以看出:在80 ℃溶出熟料时,随着时间的延长,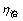 为2%左右,二次反应损失少;
为2%左右,二次反应损失少;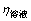 随时间延长而提高,说明原硅酸钙分解后硅主要进入溶液中,同时也说明二次反应损失不能充分反映粗液中硅浓度的变化。在60~100 ℃,随着温度升高,
随时间延长而提高,说明原硅酸钙分解后硅主要进入溶液中,同时也说明二次反应损失不能充分反映粗液中硅浓度的变化。在60~100 ℃,随着温度升高,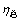 增大,但原硅酸钙中反应的硅主要进入溶液中,二次反应损失少;而在136 ℃溶出熟料时,溶液中二氧化硅浓度低,原硅酸钙分解后的硅主要以钠硅渣或钙硅渣形式存在,都会进入赤泥中,即二次反应损失大,但粗液中二氧化硅浓度低,脱硅容易。无论是时间延长,还是温度升高,二氧化硅总反应率均明显升高,即二氧化硅总反应率较好地反映了原硅酸钙的反应活性变化规律。
增大,但原硅酸钙中反应的硅主要进入溶液中,二次反应损失少;而在136 ℃溶出熟料时,溶液中二氧化硅浓度低,原硅酸钙分解后的硅主要以钠硅渣或钙硅渣形式存在,都会进入赤泥中,即二次反应损失大,但粗液中二氧化硅浓度低,脱硅容易。无论是时间延长,还是温度升高,二氧化硅总反应率均明显升高,即二氧化硅总反应率较好地反映了原硅酸钙的反应活性变化规律。
2.2.2 铝酸钠溶液αk对原硅酸钙与铝酸钠溶液反应的影响
αk为溶液中Na2O质量与Al2O3质量的比值;αk越大,溶液中以NaOH形态存在的游离Na2O就越多。铝酸钠溶液的αk对原硅酸钙与铝酸钠溶液反应的影响如图3所示。
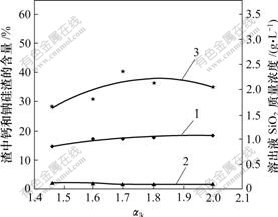
1—钙硅渣;2—钠硅渣;3—SiO2
温度80 ℃;熟料添加量60 g/L;时间60 min;
溶液成分:Al2O3 126.24 g/L;Na2Oc 25.13 g/L
图3 αk对溶出后渣中钙硅渣、钠硅渣生成和溶出液二氧化硅质量浓度的影响
Fig.3 Influence of αk on SiO2 concentration and distribution of hydrogarnet and hydrogarnet and sodium aluminosilicate hydrate
从图3可知:随着铝酸钠溶液αk的升高,溶出液SiO2浓度呈现一峰值,但整体上,SiO2质量浓度(约 2 g/L)变化幅度不大。说明在铝酸钠溶液中,游离碱浓度对原硅酸钙的分解影响不明显,这与热力学分析结果[8]相吻合。同时,钠硅渣量和钙硅渣量随反应时间的延长变化也不明显。
2.2.3 铝酸钠溶液的氧化铝质量浓度对原硅酸钙与铝酸钠溶液反应的影响
氧化铝浓度对原硅酸钙与铝酸钠溶液反应的影响如图4所示。
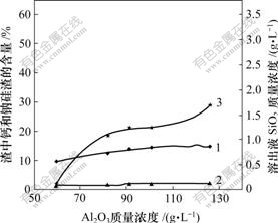
1—钙硅渣;2—钠硅渣;3—SiO2
温度80 ℃;熟料添加量60 g/L;时间60 min;
溶液其他成分:αk: 1.76;Na2Oc =0 g/L
图4 氧化铝质量浓度对溶出后渣中钙硅渣、钠硅渣生成和溶出液二氧化硅质量浓度的影响
Fig.4 Influence of Al2O3 concentration on SiO2 concentration and distribution of hydrogarnet and sodium aluminosilicate hydrate
由图4可看出,当氧化铝浓度较低时,溶出液中SiO2质量浓度也很低;但当氧化铝质量浓度超过80 g/L以后,溶出液中SiO2质量浓度增至1 g/L以上,并且随着氧化铝质量浓度的进一步提高,SiO2质量浓度也显著增大。调整液中Al2O3质量浓度从60 g/L增至120 g/L时,SiO2质量浓度从0.110 g/L到1.683 g/L,最大增幅可达15.3倍。这说明:Al(OH)4-的质量浓度越大,原硅酸钙的分解程度越大,即Al(OH)4-易与原硅酸钙发生反应。
2.2.4 碳酸钠浓度对原硅酸钙与铝酸钠溶液反应的 影响
在纯碳酸钠溶液中,原硅酸钙易被分解。在铝酸钠溶液中,碳酸钠与原硅酸钙间反应规律如图5所示。
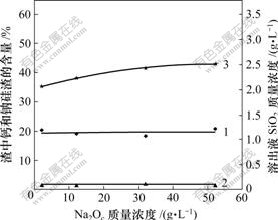
1—钙硅渣;2—钠硅渣;3—SiO2
温度80 ℃;熟料添加量60 g/L;时间60 min;
溶液成分:Al2O3 119.73 g/L; Na2Ok 127.51 g/L; αk: 1.75
图5 碳碱质量浓度对溶出后渣中钙硅渣、钠硅渣生成和溶出液二氧化硅质量浓度的影响
Fig.5 Influence of Na2Oc concentration on SiO2 concentration and distribution of hydrogarnet and sodium aluminosilicate hydrate
从图5可以看出,无论碳酸钠质量浓度多大,溶液中SiO2质量浓度都大于2.0 g/L;随着铝酸钠溶液中碳酸钠质量浓度的提高,溶出液中SiO2质量浓度略 增加。
比较图5与图4可以看出,在纯铝酸钠溶液中,Al2O3质量浓度为119.73 g/L,碳酸钠溶液质量浓度近似为零的条件下,溶出液中SiO2质量浓度为1.46 g/L;若是在含碳酸钠的铝酸钠溶液中,氧化铝质量浓度相同,碳碱质量浓度为15和55 g/L时,溶出液中SiO2质量浓度分别为2.31和2.5 g/L,这说明加入碳酸钠后铝酸钠溶液与硅酸钙的反应更显著,铝酸根离子与碳酸根离子之间存在一种类似“协同”效应,能明显促进原硅酸钙的分解。
从图2~5还可知:无论在何种溶出条件下,渣中钠硅渣含量少,且变化不明显,而钙硅渣含量多,大于钠硅渣的含量,且随反应条件的变化而变化,说明二次反应损失主要是钙硅渣的生成而引起的。
为了进一步明确原硅酸钙分解的原因,测定了原硅酸钙在不同浓度的苛性碱、碳碱、铝酸钠溶液中颗粒表面的Zeta电位进行测定,结果如图6所示。
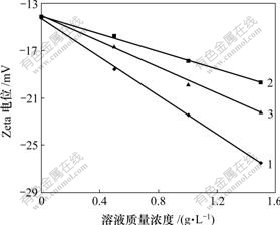
1—碳碱;2—苛性碱;3—氧化铝
温度为15 ℃;电压为10 V;电压切换时间为700 ms;电流为0.3 mA
图6 硅酸钙颗粒的表面Zeta电位随溶液成分浓度变化曲线
Fig.6 Influence of components concentration in solution on Zeta potential of 2CaO·SiO2
Zeta电位是指stern层外相对运动边界处于液体内部的电位差,能反映溶剂化层电解质浓度的变化[14]。由图6可以看出,在水溶液中,原硅酸钙颗粒的表面Zeta电位是-14.18 mV,其Zeta电位为负值的主要原因是在溶剂化层有OH-(H2O电离H+和OH-)。若加入同量1?1电解质(NaOH和NaAl(OH)4溶液),其Zeta电位均随浓度的增大其值更负,绝对值变大。说明 OH-和Al(OH)4- 更容易进入溶剂化层。相比较而言,Al(OH)4-比OH-进入溶剂化层量更多,导致Zeta电位绝对值更大,从而更有利于原硅酸钙的分解反应。而碳酸钠也使Zeta电位绝对值更大,一方面可能是其更容易进入溶剂化层引起,当然也有利于原硅酸钙的分解,另一方面可能和其价电荷(-2价)有关。
3 结 论
a. 在铝酸钠溶液中,随着反应时间的延长和氧化铝浓度的升高,原硅酸钙分解后溶出液中二氧化硅浓度明显升高,最高增幅可达9.97倍和11倍;随着反应温度的升高,因脱硅效果明显溶出液中二氧化硅质量浓度先升高后降低;随着苛性比的增大,碳酸钠质量浓度的升高,溶出液中二氧化硅浓度较高,但变化不明显;碳酸钠与铝酸钠共同存在时,会以“协同效应”促进原硅酸钙的分解。
b. 无论在何种条件下,原硅酸钙与铝酸钠溶液反应后,渣中钙硅渣含量大于钠硅渣含量,钙硅渣生成含量是钠硅渣生成含量的40倍,二次反应损失主要是因为钙硅渣的生成引起的。为表征原硅酸钙反应活性或稳定性,提出了“二氧化硅总反应率”概念,结果表明,随着反应时间延长和反应温度升高,反应率均明显升高,于136 ℃反应1 h后,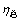 达43.06%。
达43.06%。
c. 原硅酸钙在铝酸钠、苛性碱和碳酸钠溶液的Zeta电位测定结果表明,NaAl(OH)4和Na2CO3是原硅酸钙分解的主导因素,NaOH对分解的影响比较小;原硅酸钙表面Zeta电位绝对值增大,有利于原硅酸钙的分解。
参考文献:
[1] 杨重愚. 氧化铝生产工艺学[M]. 北京: 冶金工业出版社, 1992: 230-245.
YANG Zhong-yu. The technology of alumina production[M]. Metallurgical Industry Press, 1992: 230-245.
[2] 李小斌, 徐华军, 刘桂华, 等. 氧化铝熟料溶出过程中SiO2的行为[J]. 过程工程学报, 2006, 6(3): 431-434.
LI Xiao-bin, XU Hua-jun, LIU Gui-hua, et al. Behavior of SiO2 during leaching process of alumina sinter[J]. The Chinese Journal of Process Engineering, 2006, 6(3): 431-434.
[3] Leonard J. Bauxite[M]. New York: Society of Mining Engineers of American Institute of Mining, Metallurgical and Petroleum Engineer Inc, 1984: 780-786.
[4] 陈红武, 周宗科. 烧结法熟料溶出条件对二次反应影响分析[J]. 轻金属, 2001(8): 14-16.
CHEN Hong-wu, ZHOU Zong-ke. The analysis of influences of leaching conditions on the secondary reaction occurred in clinker leaching process[J]. Light Metals, 2001(8): 14-16.
[5] 《联合法生产氧化铝》编写组. 联合法生产氧化铝: 熟料溶出与脱硅[M]. 北京: 冶金工业出版社, 1975: 5-7.
Term of《Alumina Production By Combination Process》. Alumina production by combination process: Sinter leaching and desilication[M]. Beijing: Metallurgical Industry Press, 1975: 5-7.
[6] 权 昆, 武福运. 铝酸钠溶液中硅酸二钙的分解及抑制[J]. 有色矿冶, 2005, 21(2): 27-29.
QUAN Kun, WU Fu-yun. Decomposition and restrain of the dicaicium silicate in sodium solution[J]. Non-Ferrous Mining and Metallurgy, 2005, 21(2): 27-29.
[7] 徐华军. 氧化铝熟料溶出过程中二次反应的研究[D]. 长沙: 中南大学冶金科学与工程学院, 2005.
XU Hua-jun. The research on secondary reaction during leaching process of alumina sinter[D]. Changsha: School of Metallurgical Science and Engineering, Central South University, 2005.
[8] 郭琴珍, 王 军. 二次反应对熟料中氧化铝溶出率的影响[J]. 轻金属, 2003(12): 10-12.
GUO Qin-zhen, WANG Jun. Effect of secondary reaction on the alumina leaching rate of sinter[J]. Light Metals, 2003(12): 10-12.
[9] LIU Giu-hua, LI Xiao-bin, PENG Zhi-hong, et al. Behavior of calcium silicate in leaching process[J]. Trans Nonferrous Soc, 2003, 13(1): 213-216.
[10] 李小斌, 吕卫君, 彭志宏, 等. 硅酸钙烧成与碱液溶出性质研究[J]. 矿冶工程, 2005, 25(1): 47-49.
LI Xiao-bin, L? Wei-jun, PENG Zhi-hong, et al. Researches on the sinter of calcium silicate and its digestion properties in alkaline solutions[J]. Mining and Metallurgical, 2005, 25(1): 47-49.
[11] LI Xiao-bin, ZHAO Zhuo, LIU Gui-hua, et al. Behavior of calcium silicate hydrate in aluminate solution[J]. Trans Nonferrous Met Soc China, 2005, 15(5): 1145-1149.
[12] LIU Giu-hua, LI Xiao-bin, PENG Zhi-hong, et al. Stability of caclcium silicate in basic solution[J]. Trans Nonferrous Soc China, 2003, 13(5): 1235-1238.
[13] 陈 滨, 李小斌, 徐华军, 等. 氧化铝熟料溶出过程二次反应的热力学讨论[J]. 北京化工大学学报, 2007, 34(2): 189-195.
CHEN Bin, LI Xiao-bin, XU Hua-jun, et al. Thermodynamic analysis of secondary reactions in the clinker leaching process[J]. Journal of Beijing of Chemical Technology, 2007, 34(2): 189-195.
[14] 陈宗淇, 王光信, 徐桂英. 胶体与界面化学[M]. 北京: 高等教育出版社, 2004: 159-165.
CHEN Zong-qi, WANG Guang-xin, XU Gui-ying. Colloid and interface chemistry[M]. Beijing: Higher Education Press, 2004: 159-165.
收稿日期:2008-06-15;修回日期:2008-10-20
基金项目:国家重点基础研究发展规划项目(2005CB-623702)
通信作者:刘桂华(1968-),男,湖南攸县人,教授,从事氧化铝工艺及陶瓷研究;电话:0731-8830453;E-mail: liugh303@163.com