利用无定形微硅粉生产不同硅含量硅铝合金的可能性与前景
来源期刊:中国有色金属学报(英文版)2020年第5期
论文作者:M. P. KUZ′MIN M. Yu. KUZ′MINA P. B. KUZ′MIN
文章页码:1406 - 1418
关键词:铝;硅;硅铝合金;微硅粉;二氧化硅
Key words:aluminum; silicon; silumins; microsilica; silicon dioxide
摘 要:本工作的主要目的是研究Al(l)-SiO2界面的复杂物理化学过程,开发一种利用由硅生产废料得到的无定形微硅粉生产铸造硅铝合金的新技术。开发使用无定形微硅粉生产亚共晶、共晶和过共晶硅铝合金的有效方法。将预热的无定形微硅粉用氩气流吹入铝熔体(t=900 °C),并进行剧烈混合,得到硅含量为7 wt.%的硅铝合金。先将含硅混合物(60% SiO2,40%Al + 20%3NaF·2AlF3)进行预烧结,使无定形微硅粉还原成结晶硅;再将预烧结产物进行感应熔炼,制备硅含量为21 wt.%的硅铝合金。已经证实,在压片炉料烧结过程中形成的结晶硅可以顺利被铝熔体吸收。氧化还原反应生成的氧化铝溶解在冰晶石中,铝和硅则融合并进入(合金)熔体中。使用无定形微硅粉生产硅铝合金的经济效率计算表明,该项目投资回收期短、盈利能力强。
Abstract: The main objective of this work is to research complex physical-chemical processes of Al(l)-SiO2 interface and develop a new technology for producing foundry silumins based on amorphous microsilica obtained from silicon production waste. Effective methods for producing hypoeutectic, eutectic, and hypereutectic silumins using amorphous microsilica were developed. Alloys with a silicon content of 7 wt.% were obtained by blowing preheated amorphous microsilica into the aluminum melt (t=900 °C) along with the stream of argon followed by intense mixing. Alloys with a silicon content of 21 wt.% were manufactured by induction melting of a silicon-containing mixture (60% SiO2, 40%Al + 20%3NaF·2AlF3) subjected to the presintering when the amorphous microsilica was reduced to crystalline silicon. It is found that crystalline silicon, which is formed during the roasting of the tableted burden, is smoothly absorbed by the aluminum melt. Aluminum oxide, obtained during the redox reaction, dissolves in cryolite, after which aluminum and silicon are fused together and transferred to the melt. The calculation of the economic efficiency of producing silumins using amorphous microsilica demonstrates a quick project payback period, as well as a high level of its profitability.
Trans. Nonferrous Met. Soc. China 30(2020) 1406-1418
M. P. KUZ′MIN1, M. Yu. KUZ′MINA1, P. B. KUZ′MIN2
1. Irkutsk National Research Technical University, 83 Lermontov St., Irkutsk, Russia;
2. Limited Liability Company “United Company RUSAL Engineering and Technology Center”, 4 Industrial St., Shelekhov, Irkutsk Region, Russia
Received 30 March 2019; accepted 14 April 2020
Abstract: The main objective of this work is to research complex physical-chemical processes of Al(l)-SiO2 interface and develop a new technology for producing foundry silumins based on amorphous microsilica obtained from silicon production waste. Effective methods for producing hypoeutectic, eutectic, and hypereutectic silumins using amorphous microsilica were developed. Alloys with a silicon content of 7 wt.% were obtained by blowing preheated amorphous microsilica into the aluminum melt (t=900 °C) along with the stream of argon followed by intense mixing. Alloys with a silicon content of 21 wt.% were manufactured by induction melting of a silicon-containing mixture (60% SiO2, 40%Al + 20%3NaF·2AlF3) subjected to the presintering when the amorphous microsilica was reduced to crystalline silicon. It is found that crystalline silicon, which is formed during the roasting of the tableted burden, is smoothly absorbed by the aluminum melt. Aluminum oxide, obtained during the redox reaction, dissolves in cryolite, after which aluminum and silicon are fused together and transferred to the melt. The calculation of the economic efficiency of producing silumins using amorphous microsilica demonstrates a quick project payback period, as well as a high level of its profitability.
Key words: aluminum; silicon; silumins; microsilica; silicon dioxide
1 Introduction
The development of the metallurgical industry depends on the profitability of products and the possibilities of efficient use of material and energy resources. One of the main tasks in aluminum and its alloys production is to reduce the cost of finished products, mainly due to lower energy consumption and the involvement of previously unused coproducts or waste [1,2].
The most marketable product on the aluminum consumer market is aluminum casting alloys. They allow to manufacture products with properties that fully comply the needs of final consumer [2]. Silumins are the most common aluminium casting alloys. They represent a group of aluminium alloys with the main alloying element being silicon. The demand for silumins is provided by a unique combination of their basic properties: low density, high fluidity, relatively low shrinkage, low tendency to formation of stresses and cracks, high strength properties, wear resistance and heat resistance [3,4].
Foundry silumins can be produced by electro- thermic, metallothermic, and electrolytic methods, as well as by dissolution of crystalline silicon in the aluminium melt [5-8]. The first three methods are one-stage (i.e., silumins are produced by using silica as a raw material in ore-thermal furnaces or reduction cells); however, due to the high power consumption for their implementation, difficulty of producing alloys with a given composition, as well as a possibility of alloy contamination by impurities and nonmetallic inclusions, these methods have not wide industrial applications [9,10].
Today, the main silumin ingot production method is crystalline silicon dissolution in the aluminium melt in aluminium smelters. The main advantage of this method is its high performance and the ability to produce alloys with the specified silicon content. However, this method has some significant disadvantages: high metal waste due to burning loss, low absorption of silicon fines (less than 5-6 mm), and high power consumption.
In the conditions when aluminum and silicon industries are located in close proximity, a method of producing silumin using 30% or 50% Al–Si liquid alloy can also be employed. The method consists of pouring molten silicon into a vacuum ladle which contains aluminum melt. Despite high quality alloys are produced, the method is usually associated with logistical difficulties. Thus, the production of the Al-Si system alloys under the existing layout assumes the presence of two metallurgical products: primary aluminium and crystalline silicon, which entails high economic cost and power consumption. At the same time, silicon production generates wastes, namely, the dust of the gas treatment systems of the electrothermic furnaces, consisting of (85-95) wt.% of micro- and nanoparticles of amorphous silicon dioxide. The dust yield of the silicon production ranges from 300 to 1000 kg relative to 1 t of commodity silicon.
Currently, most of metallurgical production facilities have accumulated the obtained microsilica in the slurry fields. This raises substantial economic losses, firstly, due to the costs of waste storage and burial, and, secondly, with lost profits from their industrial applications. The use of silicon dust as a source of silicon may improve efficiency of the silumin production process by partial exclusion of the energy-intensive metallurgical silicon production stage.
The price of crystalline silicon, depending on the brand, varies from 2000 to 2500 US$/t. Dust emitted from the gas systems involved in the cleaning of electrothermal furnaces can be used in the production of silumin, as it is associated with very low cost and increased energy efficiency which leads to a significant economic effect can be achieved. Moreover, recycling and use of silicon dust wastes should be seen as an important way of saving material resources and increasing environmental safety of the adjacent territories [5].
In excess of described methods and patents designed to obtain silumin, there seems to exist no technologies that can be practically applied in manufacturing sector [4-13]. Hence, the purpose of this work is to research a complex of physical- chemical processes of Al(l)-SiO2 interface and develop a technology for producing low- temperature silumin (in accordance with GOST 1583—93) based on the amorphous microsilica obtained from the silicon waste production.
2 Experimental
The calculation of the thermodynamic probability of silicon reduction by aluminum from amorphous microsilica, as well as calculations of the stability of chemical compounds formed during the interaction of silicon oxide with technical aluminum impurities, was carried out over a wide temperature range based on data about the basic physicochemical parameters (standard enthalpy of formation and Gibbs free energy), as well as using the programs “PANDAT”, “CHS Chemistry 5” and “SELECTOR”. Since the initial data on thermodynamic function standard values for a number of the considered chemical compounds did not exist (in the literature and used software) or did not correlate with each other, well-known and adapted methods of approximate calculations were used [14-16].
Commercial purity aluminum with the following contents of the main impurities (wt.%: 0.10 Si, 0.112 Fe, 0.02 Mg, 0.023 Mn, 0.015 Cu, 0.031 Zn, 0.001 Ga, 0.001 Ti, 0.001 V) was used as the base metal for the laboratory research on silumin production with application of amorphous silica.
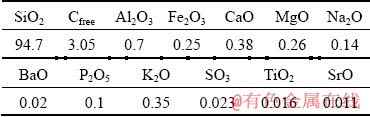
Amorphous microsilica was obtained from various parts of the gas cleaning system of JSC “silicon” (Shelekhov, Irkutsk Region, Russia): at the entrance to the gas removal system, after the cyclones at the entrance to the gas cleaning unit, and after application of the gas cleaning system. The chemical composition of the used dust is presented in Table 1.
If the obtained dust contained a high content of carbon and other impurity elements, the flotation was used to enrich it. Flotation of micro- and nanosized particles of SiO2 was carried out in a laminar flow of pulp (with the least number of elementary flotation cycles) using a pneumo- hydraulic aerator [17,18]. Pine oil (100 mg/L3) was used as a foaming agent. Microsilica particles were concentrated in the flotation tail. The resulting suspension was coagulated and flocculated, and the resulting product consisted of 94.7% SiO2 particles.
Table 1 Chemical composition of silicon production gas cleaning system dust after flotation enrichment (wt.%)
The introduction of silica into molten aluminum was accomplished by different methods.
(1) To the melt surface.
(2) To the bottom of the crucible followed by melt pouring.
(3) Using a mechanical bolder (in a bell).
(4) By portions into a funnel formed with melt continuous stirring.
(5) In the form of master alloy as pressed tablets of the composition aluminum powder: SiO2.
(6) Placing particles in melt in a semiliquid condition.
(7) By blowing SiO2 particles as well as the stream of argon into the melt.
(8) Induction melting of the previously prepared mixture (60%SiO2-40%Al-20%3NaF·
2AlF3) under a layer of cryolite.
There are several factors that complicate silumin production with the use of amorphous microsilica by its simple introduction in the aluminium melt.
(1) Low contact area of the microsilica powder with aluminum melt.
(2) Presence of gas films on the surface of the microsilica particles, preventing effective interaction at the interface of the liquid and solid phases.
(3) Presence of (40-50) vol.% of air in the powder, reducing its density, heat capacity, and thermal conductivity.
(4) Presence of a developed surface and high surface energy in microsilica particles.
In this regard, four methods were characterized by low efficiency, since their implementation failed to maintain the constant contact in the Al(l)–SiO2 system as well as the transformation of silicon to aluminum melt.
The use of preformed ligatures ensures reduction of silicon; however, the process occurs directly in the contact zone of the melt with the surface of the tablet as well as in the tablet volume (upon the interaction of aluminum powder particles and microsilica) [2]. The introduction of microsilica into the solid-liquid melt allows to obtain alloys with a silicon content exceeding 3 wt.%, as the aluminum melt in solid-liquid state is characterized by high viscosity which, in turn, facilitates the mixing in of dispersed particles [15,16]. The greatest efficiency was demonstrated by the methods of injecting silicon dioxide into an aluminum melt in the argon flow, as well as induction melting of a previously prepared silica-containing mixture. Silica injection into an aluminum melt was carried out in the laboratory setup shown in Fig. 1.
1000 g aluminium was placed in the crucible made of boronsiliconized graphite. The heat took place in top-charging muffle shaft furnace. The metal was heated to a temperature of 900 °C, melted, and then amorphous microsilica (previously subjected to heat treatment inside a heated bin) was introduced in the metal together with the inert gas stream. In connection with low wettability of microsilica particle, its introduction into the aluminum melt was performed with the excess when compared with the stoichiometric ratio. For example, in order to fabricate the hypoeutectic alloy, 200 g SiO2 was introduced into the melt (against 137 g (6% Si) and 161 g (7% Si), necessary according to stoichiometry). Figure 2 presents the SEM image of microsilica particles with a spherical shape. It is seen that the size of SiO2 particles varies within a wide range (from 100 nm to 5 μm).
To improve wettability of the microsilica particles and prevent formation of agglomerates, before introduction into the aluminium melt, they were subjected to the heat treatment at a temperature of 200-300 °C. Likewise to solve the wettability problem the melt was alloyed with magnesium. Magnesium was introduced into the melt in the form of MG-90 magnesium alloy. The use of magnesium in the silumin production process was conditioned by the fact that it can act as a surface-active additive in the melt for removal of oxygen from the surface of dispersed particles, decline of the surface tension of the molten aluminium and reduction of the energy of interfacial interaction between the solid and liquid phases [8,11-13].
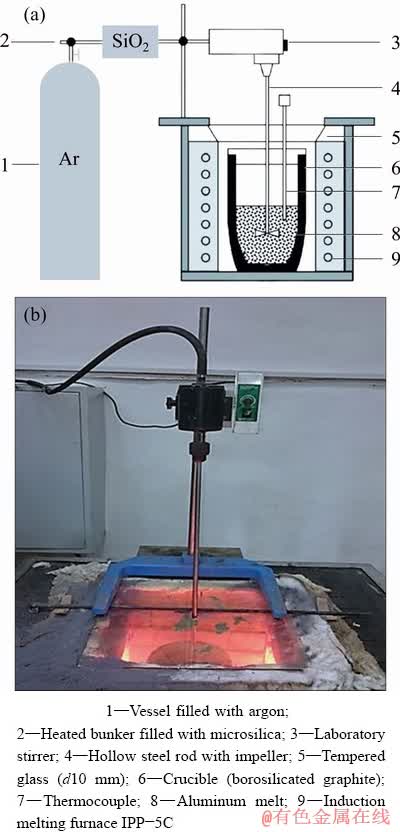
Fig. 1 Scheme (a) and photo (b) of laboratory setup for producing casting silumins
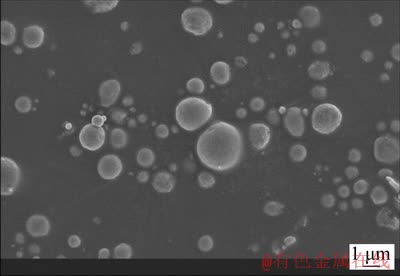
Fig. 2 SEM image of SiO2 particles
To increase the transition degree of silicon into the aluminum melt, the technology of producing silumins using a previously prepared mixture consisting of silicon dioxide, aluminum powder, and cryolite was tested. The purpose of the preliminary preparation of the mixture was the reduction of amorphous microsilica to crystalline silicon. The procedure was performed sequentially in several stages:
(1) Preparation of the mixture consisting of silicon dioxide, aluminum powder (or aluminum chips) and low-modular cryolite in the ratio: 60%SiO2-40%Al-20%3NaF·2AlF3. The ratio in the mixture of silicon dioxide and powdered aluminum is defined in accordance with the stoichiometric calculation 3SiO2 (180 g/mol) + 4Al (108 g/mol) → 2Al2O3 (204 g/mol) + 3Si (84 g/mol). Cryolite is essential to the mixture as it creates an atmosphere of reducing roasting and shifting the reaction equilibrium towards the formation of the end products.
(2) Pressing the mixture into a tablet (with a force of 100 kN).
(3) Tablet burning at the temperature of 800 °C for 30 min.
(4) Grinding the sintered material (using planetary ball mill RM 100) to a fraction of 100 μm, which ensures the maximum contact degree in the Al-Si system.
Upon the completion of the preparatory operations, the mixture containing silica in crystalline form was loaded into a crucible of borosilicate graphite. Solid aluminum was placed in the crucible, filled with a layer of cryolite (5 cm), corrected with aluminum fluoride to reach the melting point (t=800 °С). The melting was carried out in the induction furnace in the temperature range of 850-900 °C. After the metal was melted, it was intensively mixed mechanically for 5 min. To remove the by-products of the reduction reaction between silicon and aluminum, as well as non-metallic inclusions, gases and oxide films, metal was treated with the covering-refining flux before metal casting.
SiO2 particles were mixed in the melt using IKA EUROSTAR 200 Control P4 laboratory mixer (Germany) with a rotation speed range of 0-530 r/min. The silicon contents in the metal before and after the experiments were determined using optical emission spectrometer with the spectrum spark excitation source SPECTROLAB manufactured by SPECTRO Analytical Instruments (Germany). To study the phase composition of the impurities included in the composition of the tested samples, the X-ray diffractometry was used with application of X-ray diffractometer XRD-7000 manufactured by Shimadzu. The metal samples were studied in the 2θ range from 10° to 70°.
The microstructure of the samples was studied using scanning electron microscope JIB-4500 multibeam manufactured by JEOL, equipped with energy-dispersive detector X-Max manufactured by Oxford Instruments. The microstructure of the samples was studied in the modes of secondary and back-scattered electrons.
For the thorough analysis of the microstructure of the obtained silumin samples, metallographic examinations were carried out using an Olympus GX–51 inverted optical microscope (Japan). The macrostructure of the samples was studied using stereoscopic microscope SZX 16 manufactured by Olympus (Japan). Quantitative analysis of the parameters of macrostructure was performed using an image analyzer SIAMS 700 by SIAMS Ltd. (Russian).
3 Results and discussion
The study of the possibility for silumin production by introduction of SiO2 particles in the aluminium melt is impossible without a preliminary thermodynamic analysis. We determined the thermodynamic possibility for proceeding the process of silicon reduction by aluminium from amorphous silica (4Al+3SiO2=2Al2O3+3Si) in the temperature range between 298 and 1600 K. The values of Gibbs free energy in the investigated temperature range were calculated with a step of 100 K. The calculations were carried out upon considering polymorphic transformations of silica (quartz—tridymite—cristobalite) in accordance with the existing data [10,19,20].
It was found that Gibbs free energies have negative values throughout the investigated temperature range (Fig. 3(a)).
ΔG-T curve shifts to the region of less negative values with temperature rise. This shows, firstly, a possibility of amorphous silica reduction at the temperatures above the aluminium melting temperature, and, secondly, the reduced intensity of this process proceeding at subsequent higher temperatures. It was also discovered that polymorphic transformations of silica, which occur at the temperatures of 1143 and 1543 K, do not affect the smoothness of the ΔG-T curve.
In our opinion, the reduction of silicon by aluminum in a temperature range of 690-900 °C occurs due to the simultaneous run of the following two processes in the surface layer of the Al(l)-SiO2 system.
(1) Separation of SiO2 from the surface of microspheres and its transfer into the aluminum melt with the subsequent dissolution. The authors of Refs. [21-23] considered the dissolution mechanism and kinetics of quartz sand in the aluminum melt. In the case with microsilica, this process can be substantially accelerated due to its amorphous state and particle sizes.
(2) The run of the main chemical reaction of reduction of silicon by aluminum (3SiO2 + 4Al = 2Al2O3 + 3Si) and side reactions (the interaction of aluminum and microsilica with impurities and alloying additives).
It should be noted that the dissolution of refractory materials in low-melting ones is widespread in the practice of metallurgical production. The closest example is the dissolution of solid crystalline silicon (tm=1414 °C) in aluminum in the course of the industrial production of silumins (the melt temperature does not exceed 750 °C).
To determine the effect of alloying additives and impurities contained in technical aluminum and silicon dust on the process of silicon reduction, the analysis of physicochemical parameters characterizing the thermodynamic stability of the compounds formed in a wide temperature range was employed as the main research method. Among the chemical compounds formed in the Al-SiO2 system, Al2O3, Al4C3, SiC, Mg2Si, and MgO were proved to be of the utmost interest with regard to their impact on the interfacial interaction process in the system.
The calculations were carried out within a wide range of temperatures. The standard temperature of 298 K was picked as the initial one while the maximum melting point of the chemical compound (~2000-3000 K) was to be the final one. Al4C3 and SiC were chosen as silicon production dust in its initial state containing up to 25 wt.% of C and after flotation treatment up to 3 wt.% C. The formation of magnesium compounds (Mg2Si, MgO) was studied as magnesium was used as a surfactant to increase wettability in the Al(l)-SiO2 system during the experimental research.
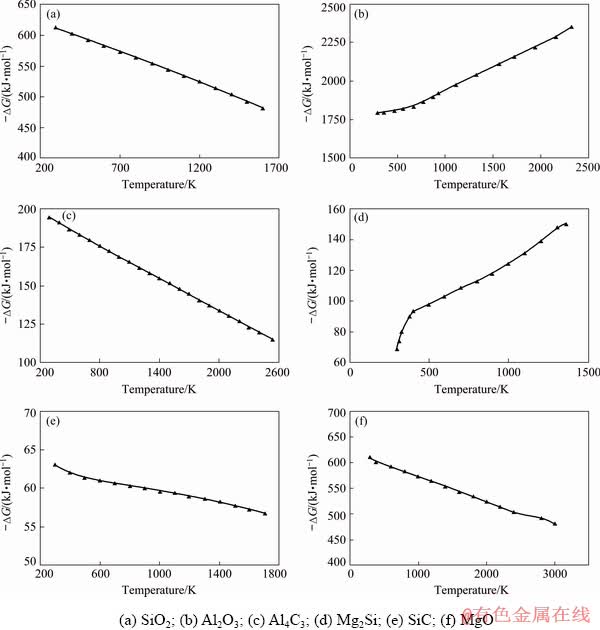
Fig. 3 Changes of Gibbs free energy of different compounds
According to the calculations, the Gibbs free energies in all compounds have negative values in the studied temperature ranges under study (Figs. 3(b-f)). This phenomenon suggests that during the production of silumins using amorphous microsilica as a source of silicon in the temperature range of 690-900 °C, additional refining of the aluminum melt with the use of fluxes is deemed necessary. The stability of the MgO chemical compound indicates the possibility of using magnesium as a surface-active additive that helps to remove oxygen from the surface of the dispersed particles and reduce silicon from silicon dioxide, along with aluminum.
3.1 Characterization of silumins prepared by blowing amorphous silica into aluminum melt in argon stream
The alloy samples obtained in the course of experimental studies were examined by optical emission spectroscopy, scanning electron spectro- scopy, optical microscopy, as well as by X-ray diffractometry.
The results of optical emission spectroscopy demonstrate that in the resulting alloy compared to the original aluminum, the silicon content increased from 0.123 wt.% to 6.955 wt.% (Table 2).
Table 2 Contents of impurities in original aluminum and resulting alloy prepared by blowing amorphous silica into aluminum melt in argon stream
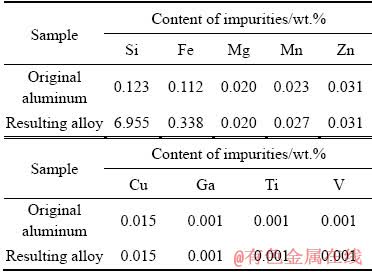
The XRD pattern of the sample belonging to the produced alloy exhibits the peaks corresponding to aluminium, silicon, and SiO2 (Fig. 4).
The peaks corresponding to the metallic aluminium (2θ=44.7°, 65.2°) and crystalline silicon (2θ=26.3°, 38.9°, 48.3°, 57.1°) possess the highest intensity. The peaks belonging to silicon dioxide are present in the 2θ range from 20° to 30°. Their weak intensity is due to predominance of silicon in the crystalline form in the structure of the produced alloy.
The microstructure studies of the original aluminum and the resulting alloy samples confirmed the increase in the silicon content in the resulting alloy.
The energy-dispersive detector of the microscope during scanning of the electron beam made it possible to record the intensity of the characteristic X-ray lines of the elements and, consequently, to receive their two-dimensional map of distribution. The distribution maps for the basic elements Al and Si are presented in Fig. 5.
The distribution map for silicon of the produced alloy sample clearly shows presence of silicon inclusions at the aluminium grain boundaries (Fig. 5(d)), which are virtually absent in the original metal (Fig. 5(b)). This confirms the results obtained during spectral analysis of the samples.
Fig. 4 XRD pattern of alloy prepared by blowing amorphous silica into aluminum melt in argon stream in 2θ range of 10°-70°
The produced alloy microstructure corresponds to the hypoeutectic silumins and consists of dendrites of a solid solution of silicon in aluminium (α(Al)) and eutectic α(Al)+Si, located in the interdendritic space (Fig. 6(b)). In the analysis of alloy microstructure no iron-containing phases were detected. It is possible to observe a cross- section (macrograph) of the produced ingot in Fig. 6(a). It should be noted that the ingot microstructure and macrostructure are characterized by availability of a scattered porosity, the occurrence of which is due to the presence of SiO2 particles, which have not started interacting with aluminium, in the volume of the crystallized metal.
Despite the development of a scattered porosity in the casting (density index of 4.9%), the conducted research showed the possibility and the prospects of silumin production with use of amorphous silica as the silicon source. A preliminary thermal treatment of amorphous silica had a decisive influence on the intensity of interaction in Al(l)-SiO2 system; it allowed removal of gas films from the surface of the particles, as a result, to reduce the surface tension of the melt, as well as to reduce the energy of interfacial interaction between the solid and liquid phases. Acceleration of the process of silicon reduction from its oxide was realized by introduction of silica particles together with a stream of argon and their subsequent vigorous stirring.
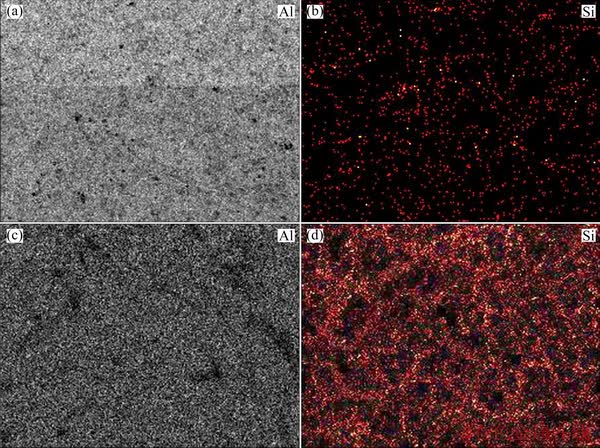
Fig. 5 Distribution maps for aluminum (a, c) and silicon (b, d) of original aluminum (a, b) and resulting alloy (c, d) samples
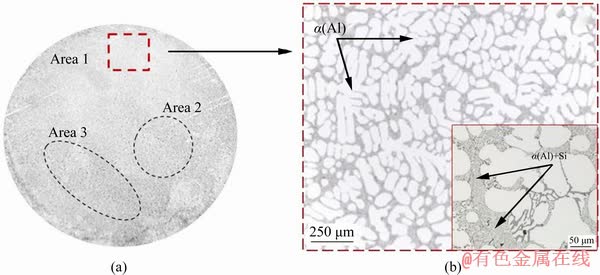
Fig. 6 Macrostructure (a) and microstructure (b) of resulting alloy (Area 1 has exponential microstructure; Areas 2 and 3 have diffusive porosity)
3.2 Characterization of silumins prepared by induction melting of previously prepared silica-containing mixture
The studies demonstrated that upon injecting amorphous silica into aluminum melt in the argon stream, only can hypoeutectic alloys be obtained. Such a phenomenon can be explained by physical properties of the silica (amorphous structure, particle size, density) and the technological design of the process (cooling of the melt temperature during its long purging with argon). Further, it was discovered that to obtain eutectic and hyper- eutectic silumins, preliminary preparation of the silicon-containing mixture, including pressing it (60%SiO2-40%Al-20%3NaF·2AlF3) into tablets, subjecting it to the heat-treatment (t=800 °С), and the subsequent grinding of the sintered mass to a fraction of 100 μm is required.
The sintering temperature is determined to create conditions for the solid phase interaction. The grinding of the sintered mass is supposed to increase the area of its contact with the aluminum melt. As a result of sintering of the silica-containing mixture, practically all of its microsilica (more than 95 wt.%) is reduced to crystalline silicon. The XRD pattern of the sintered silica-containing mixture exhibits the absence of the peaks corresponding to silicon dioxide (Fig. 7).
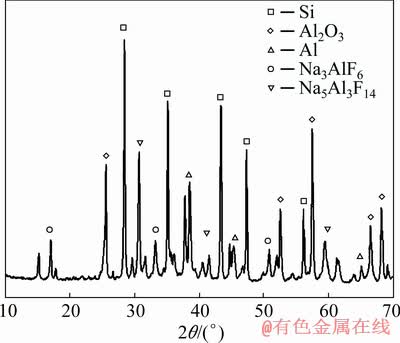
Fig. 7 XRD pattern of sintered silica-containing mixture
The peaks corresponding to crystalline silicon (2θ=28.3°, 34.9°, 43.6°, 57.1°) and aluminum oxide (2θ=25.4°, 53.2°, 57.3°, 66.2°, 67.8°) possess the highest intensity. Also, the XRD pattern exhibits the peaks corresponding to aluminum and two individual crystalline phases of the NaF-AlF3 system (cryolite and chiolite).
The density of the resulting mixture is 1.4-1.6 g/cm3. This value does not exceed the density of the molten low-modulus cryolite, which makes it possible to create a mechanical mixture of these components. In the cryolite environment, aluminum oxide is dissolved, and aluminum and silicon are melted. For the completion of the process, the optimum content of Al2O3 in cryolite amounting to (10-11) wt.% was ensured [9,24,25].
Crystalline silicon formed during tablet burning (as a result of reactions in solid phases) is smoothly absorbed by the aluminum melt.
Aluminum oxide, obtained as a result of oxidation- reduction reaction, is dissolved in cryolite. It is found that the content of alumina in cryolite (19.7 wt.%) provides the presence of an equilibrium system with a eutectic temperature of 962 °C and its complete dissolution. After that, aluminum and silicon are melted.
The dissolution of alumina in cryolite can be explained by the exchange of F- and О2- ions between the anions of 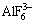 of molten cryolite and the alumina lattice. The cryolite Al3+ cations characterized by the strong field, pull the О2- out of the alumina lattice. The exchange causes the breakage of the alumina crystal lattice which, in turn, leads to the dissolution of the alumina. Thus, aluminum cations Al3+, i.e., the components of the cryolitic complexes
of molten cryolite and the alumina lattice. The cryolite Al3+ cations characterized by the strong field, pull the О2- out of the alumina lattice. The exchange causes the breakage of the alumina crystal lattice which, in turn, leads to the dissolution of the alumina. Thus, aluminum cations Al3+, i.e., the components of the cryolitic complexes 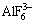 and
and 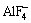 serve as “solvents” of alumina in cryolite.
serve as “solvents” of alumina in cryolite.
In the experimental research, a crucible made of borosilicated graphite was loaded with 780 g of the mixture (30% crystalline silicon, 50% aluminum oxide, and 20% cryolite), 1000 g of A7 grade aluminum, and 500 g of cryolite. As a result of experimental melting, a hypereutectic alloy was obtained (m=1340 g) with the silicon content exceeding 20 wt.%. That was confirmed by the results of optical emission spectroscopy (Table 3).
The results demonstrated that practically all crystalline silicon and aluminum that were in the mixture, turned into the melt. The use of a laboratory stirrer with a steel impeller can be accounted for the increase in the iron content in the resulting alloy. To reduce the iron content in the alloy and prevent the reduction of the complex of mechanical and casting properties, the steel impeller can be replaced with titanium one, and mixing can be carried out by an electromagnetic method. Cryolite from smelting was discharged separately for further recycling. Thus, the possibility of almost complete absorption of silicon from the composition of amorphous silicon dioxide in an aluminum melt, and the production of hypoeutectic and hypereutectic silumins, corresponding to GOST 1583—93 were experimentally proved.
Table 3 Contents of impurities in original aluminum and resulting alloy prepared by induction melting of previously prepared silica-containing mixture
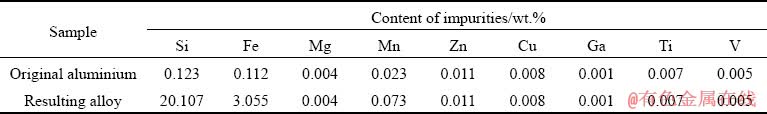
The XRD pattern of the produced alloy exhibits the peaks corresponding to aluminium, silicon, and intermetallic compounds based on iron (Fig. 8).
Fig. 8 XRD pattern of alloy prepared by induction melting of previously prepared silica-containing mixture in 2θ range of 10°-70°
The peaks corresponding to metallic aluminium (2θ=26.3°, 28.1°, 32.4°, 38.5°, 57.9°, 68.1°) and crystalline silicon (2θ=35.2°, 44.7°, 47.3°, 56.2°) possess the highest intensity. Due to the high degree doping of the alloy, peaks of the iron-containing phase β-FeSiAl5 (2θ=41.8°, 45.9°, 50.7°, 65.8°) are shown on the diffractogram: all in accordance with the existing data [26-38].
The reduction of silicon from its oxide in a mixture with aluminum and cryolite, along with a direct aluminothermic reaction, can also occur during its interaction with aluminum fluoride by the reaction of 3SiO2+2(3NaF·2AlF3)=3SiF4+2Al2O3+ 6NaF followed by reduction of silicon from SiF4 and Al: 3SiF4+4Al=4AlF3+3Si [20,32,39-44]. In this case, part of the silicon can be lost due to the release of silicon tetrafluoride in the gas phase. However, in our case, based on the data of XRD analysis, it is found that the bulk silicon goes into the melt. Emission to the gas phase is insignificant and does not exceed the established MPC values (0.5 mg/m3).
Optical electron microscopy of the obtained alloy confirms that the microstructure of the obtained alloy corresponds to hypereutectic silumin (Fig. 9).
The macrostructure of the resulting alloy is homogeneous and demonstrates the absence of gas porosity in the mold (density of 2.3%). The sample structure is fine-grained with average grain size of 0.85 mm. Microstructure consists of the following components: crystals of the primary silicon in the form of faceted plates (average size of 10 μm); eutectic silicon crystals; fine equiaxed particles of the secondary silicon (size of up to 1 μm); large inclusions of iron-containing phases β-FeSiAl5, which are complex 3D plate structures (insert in Fig. 9(с)) and on the polished 2D specimen having the form of plates with 1-8 μm in thickness and 10-50 μm in length. Second-order dendritic cells in the silumin structure are not present due to the high iron content.
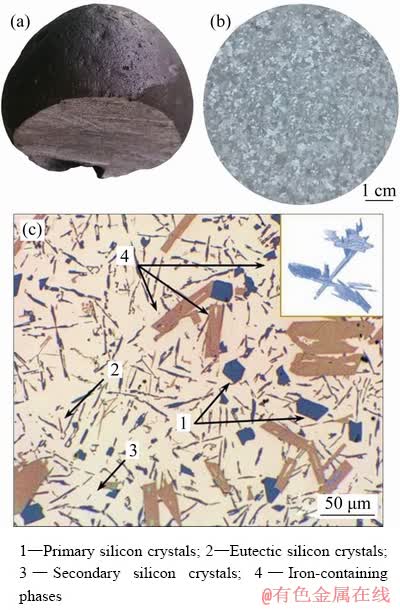
Fig. 9 Appearance (a), macrostructure (b) and micro- structure (c) of obtained hypereutectic Al-Si alloy
3.3 Economic efficiency of proposed methods
In this study, the economic efficiency of the industrial implementation of the developed methods for producing foundry silumins was also calculated.
During the calculation, the sales of the alloy were taken equal to 15×106 US$ (8036 t/year). The average price of crystalline silicon is 2250 US$/t. The price of microsilica is 25 US$/t, but since the silicon content in microsilica is about 50%, 25 must be multiplied by two. In this regard, the cost of silicon, which is a part of SiO2 will be 50 US$/t. Thus, the price difference for crystalline silicon and microsilica is 2200 US$.
The potential additional profit (P) (not taking into consideration the costs of technologies organizing) per one ton of 7 wt.% Si alloy will be
P=2200×0.07=154 US$.
Taking into consideration the planned volume of aluminum alloys production, the annual potential profit (not taking into consideration the costs of technologies organizing) when using new technologies for producing silumins will be
P=154×8036=1237544 US$.
Estimated costs for the development and implementation of each technology for producing silumins (project documentation development; manufacturing of a device for feeding and introducing SiO2 into an aluminum melt; materials and equipment for devices; construction and erection, balancing and commissioning work) will be 443478 US$.
Since in the case of using microsilica, silicon is in a bound state, the cost of silicon reduction process by aluminum was determined. The cost of this process is 52 US$/t and includes such components as material costs (components: replaceable rotors made of borosilicate graphite, blades for removing slag, and thermocouples; auxiliary materials: fluxes, and electricity), labor costs, social costs, depreciation of fixed assets and other expenses (expenses for the removal of Al2O3 and its secondary processing in cell, repair and maintenance of equipment, training and education of personnel).
Additional profit is defined as the difference between the sales proceeds of the alloy obtained by traditional and new technologies, and is 102 US$/t. Thus, taking into consideration the total volume of alloy production (by 15×106 US$), the additional profit will be
P=102×8036 = 819672 US$.
Based on the amount of capital costs and profits obtained during the implementation of the project, it is possible to calculate its payback period (PB):
PB=443478/819672=0.54 (≈0.5 year).
Thus, in the case of industrial implementation of technologies for producing silumins using amorphous microsilica, they will have a quick payback and will increase the economic efficiency of foundry.
4 Conclusions
(1) The calculations of Gibbs energy of the silicon reduction process by aluminium from its oxide (T=298-1600 K), allowed to establish a possibility of producing alloys of the Al–Si system using microsilica.
(2) In the course of determining the influence of alloying additives and impurities on the reduction of silicon, it was demonstrated that magnesium can be used as a surfactant to remove oxygen from the surface of dispersed particles and to reduce silicon from the oxide.
(3) The experimental studies demonstrated the prospects for production of hypoeutectic alloys by introducing the amorphous silica together with argon stream into the aluminium melt (t=900 °C). The necessary assimilation degree of SiO2 particles by the molten aluminium was achieved due to their pre-heat treatment.
(4) Effective method was proposed for obtaining branded alloys (preeuthectic, eutectic and hypereutectic) by induction melting of a silica-containing mixture, during the preliminary sintering of which amorphous microsilica was reduced to crystalline silicon. The method allowed to obtain silumins corresponding to GOST 1583—93, with a silicon content of 20.1 wt.%.
(5) The calculation of the economic efficiency of producing silumins using amorphous microsilica demonstrated a quick project payback period (≈0.5 year), as well as a high level of its profitability (819672 US$).
Acknowledgments
The research was carried out in the Framework of State Task of the Ministry of Education and Science of Russia (Project No. 0667-2020-0037 “Development of the scientific foundations of effective processes for obtaining valuable elements, Substances and materials from waste and man- made and natural multicomponent mixtures, including methods of design and technological support”).
References
[1] KUZ'MIN M P, KUZ'MINA M Y, KUZ'MINA A S. Production and properties of aluminum-based composites modified with carbon nanotubes [J]. Materials Today: Proceedings, 2019, 19: 1826-1830.
[2] KUZ'MIN M P, LARIONOV L M, KONDRATIEV V V, KUZ'MINA MY, GRIGORIEV V G, KNIZHNIK A V, KUZ'MINA A S. Fabrication of silumins using silicon production waste [J]. Russian Journal of Non-Ferrous Metals, 2019, 60: 483-491.
[3] KUZ'MIN P B, KUZ'MINA M Y. About the production of ingot of primary strontium modified silumins [J]. Foundry Production, 2014, 8: 2-5.
[4] ZHENG Zhi-kai, JI Yong-jian, MAO Wei-min, YUE Rui, LIU Zhi-yong. Influence of rheo-diecasting processing parameters on microstructure and mechanical properties of hypereutectic Al-30%Si alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1264-1272.
[5] WEI Dong-hui, GAO Shuai-bo, KONG Jian, JIN Xing, JIANG Sheng-nan, ZHOU Shi-bo, ZHANG Yan-xin, YIN Hua-yi, XING Peng-fei. Recycling silicon from silicon cutting waste by Al-Si alloying [J]. Journal of Cleaner Production, 2020, 251: 119647.
[6] STEENT A H, HELLAWELL A. Structure and properties of aluminium-silicon eutectic alloys [J]. Acta Metallurgica, 1972, 20: 363-370.
[7] PIETROWSKI S. Characteristic features of silumin alloys crystallization [J]. Materials & Design, 1997, 18: 373-383.
[8] JIANG Bo, JI Ze-sheng, HU Mao-liang, XU Hong-yu, XU Song. A novel modifier on eutectic Si and mechanical properties of Al-Si alloy [J] Materials Letters, 2019, 239: 13-16.
[9] BELOV N A, SAVCHENKO S V, HVAN A V. Phase composition and structure of silumins [M]. Moscow: MISiS, 2008.
[10] BELOV N A. Phase composition of aluminum alloys [M]. Moscow: MISiS, 2009.
[11] SREE MANU K M, SREERAJ K, RAJAN T P D, SHEREEMA R M, PAI B C, ARUN B. Structure and properties of modified compocast microsilica reinforced aluminum matrix composite [J]. Materials & Design, 2015, 88: 294-301.
[12] PAI B C, RAMANI G, PILLAI R M, SATYANARAYANA K G. Role of magnesium in cast aluminium alloy matrix composites [J]. Journal of Materials Science, 1995, 30: 1903-1911.
[13] GOWRI SHANKAR M C, JAYASHREE P K, KINI ACHUTHA U, SHARMA S S. Effect of silicon oxide (SiO2) reinforced particles on ageing behavior of Al-2024 alloy [J]. International Journal of Mechanical Engineering and Technology, 2014, 5: 15-21.
[14] BEGUNOV A I, KUZ′MIN M P. Thermodynamic stability of intermetallic compounds in technical aluminum [J]. Journal of Siberian Federal University: Engineering and Technologies, 2014, 7: 132-137.
[15] KUZ′MIN M P, KONDRAT′EV V V, LARIONOV L M, KUZ′MINA M Y, IVANCHIK N N. Possibility of preparing alloys of the Al-Si system using amorphous microsilica [J]. Metallurgist, 2017, 61: 86-91.
[16] KUZ′MIN M P, KONDRATIEV V V, LARIONOV L M. Production of Al-Si alloys by the direct silicon reduction from the amorphous microsilica [J]. Solid State Phenomena, 2018, 284: 647-652.
[17] KONDRATIEV V V, GOVORKOV A S, KOLOSOV A D, GOROVOY V O, KARLINA A I. The development of a test stand for developing technological operation flotation and separation of MD2. The deposition of nanostructures MD1 produce nanostructures with desired properties [J]. International Journal of Applied Engineering Research, 2017, 12: 12373-12377.
[18] KONDRATIEV V V, NEBOGIN S A, GOROVOY V O, SYSOEV I A, KARLINA A I. Description of the test stand for developing of technological operation of nano-dispersed dust preliminary coagulation [J]. International Journal of Applied Engineering Research, 2017, 12: 12809-12813.
[19] ROBIE A R, HEMINGWAY B S. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures [M]. Washington: United States Government Printing Office, 1995.
[20] AILER R. Chemistry microsilica [M]. Moscow: Mir, 1982.
[21] RAFAL'SKIJ I V, NEMENENOK B M. Physicochemical interaction of the components of the Al/SiO2 system in metallurgical process for the synthesis of foundry dispersion- strengthened aluminum alloys [J]. Lit'jo i Metallurgija, 2017, 2: 31-39. (in Russia)
[22] YAN Wei, CHEN Wei-qing, ZHANG Sen-lin, LI Bing, LI Jing. Evolution of solidification structures and mechanical properties of high-Si Al alloys under permanent magnetic stirring [J]. Materials Characterization, 2019, 157: 109894.
[23] SHU Rui, JIANG Xiao-song, LI Jing-rui, SHAO Zhen-yi, ZHU De-gui, SONG Ting-feng, LUO Zhi-ping. Microstructures and mechanical properties of Al-Si alloy nanocomposites hybrid reinforced with nano-carbon and in-situ Al2O3 [J]. Journal of Alloys and Compounds, 2019, 800: 150-162.
[24] HU Shao-dong, DAI Yan-chao, GAGNOUD A, FAUTRELLE Y, MOREAU R, REN Zhong-ming, DENG Kang, LI Chuan-jun, LI Xi. Effect of a magnetic field on macro segregation of the primary silicon phase in hypereutectic Al-Si alloy during directional solidification [J]. Journal of Alloys and Compounds, 2017, 722: 108-115.
[25] SHABESTARI S G, PARSHIZFARD E. Effect of semi-solid forming on the microstructure and mechanical properties of the iron containing Al-Si alloys [J]. Journal of Alloys and Compounds, 2011, 509: 7973-7978.
[26] CHEN Ji-qiang, LIU Chao, WEN Feng, ZHOU Qiong-yu, ZHAO Hong-jin, GUAN Ren-guo. Effect of microalloying and tensile deformation on the internal structures of eutectic Si phase in Al-Si alloy [J]. Journal of Materials Research and Technology, 2020, 105: 303-318.
[27] ZHAO Y, LIU H B, ZHAO C Y. Experimental study on the cycling stability and corrosive property of Al-Si alloys as phase change materials in high-temperature heat storage [J]. Solar Energy Materials and Solar Cells, 2019, 203: 110165.
[28] KANG N, EL MANSORI M. A new insight on induced- tribological behaviour of hypereutectic Al-Si alloys manufactured by selective laser melting [J]. Tribology International, 2019, 134. 105751.
[29] KUZ′MIN M P, LI X Y, KUZ’MINA M Y, BEGUNOV A I, ZHURAVLYOVA A S. Changing the properties of indium tin oxide by introducing aluminum cations [J]. Electrochemistry Communications, 2016, 67: 35-38.
[30] BASAK C B, MEDURI A, HARI BABU N. Influence of Ni in high Fe containing recyclable Al-Si cast alloys [J]. Materials & Design, 2019, 182: 108017.
[31] LIU M, ZHENG R, XIAO W, YU X, PENG Q, MA C. Concurrent enhancement of strength and ductility for Al-Si binary alloy by refining Si phase to nanoscale [J]. Materials Science and Engineering A, 2019, 751: 303-310.
[32] ZENKOV E V, TSVIK L B. Stress-strain state of prismatic samples with hollow chamfers [J]. Russian Engineering Research, 2013, 33: 562-565.
[33] KUZ′MIN M P, LARIONOV L M, KONDRATIEV V V, KUZ′MINA M Y, GRIGORIEV V G, KUZ′MINA A S. Use of the burnt rock of coal deposits slag heaps in the concrete products manufacturing [J]. Construction and Building Materials, 2018, 179: 117-124.
[34] LIN Chong, WU Shu-sen, Lü Shu-lin, ZENG Jin-biao, AN Ping. Dry sliding wear behavior of rheocast hypereutectic Al-Si alloys with different Fe contents [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 665-675.
[35] ZENKOV E V, TSVIK L B. Increasing the reliability the combined criteria of the static strength of a material of complexly loaded deformable structures [J]. Materials Physics and Mechanics, 2018, 40: 124-132.
[36] KONDRAT′EV V V, ERSHOV V A, SHAKHRAI S G, IVANOV N A, KARLINA A I. Formation and utilization of nanostructures based on carbon during primary aluminum production [J]. Metallurgist, 2016, 60: 877-882.
[37] FEDOROV S N, BAZHIN V Y. Development of mechanical properties of aluminum-silicon alloys [J]. Smart Nanocomposites, 2015, 6: 199-202.
[38] GORLANOV E S, BAZHIN V Y, FEDOROV S N. Low-temperature phase formation in a Ti-B-C-O system [J]. Tsvetnye Metally, 2017, 8: 76-82.
[39] KUZ′MIN M P, LARIONOV L M, KUZ′MINA M Y, KUZ′MINA A S. Synthesis of hypereutectic silumins using amorphous silicon dioxide [J]. Tsvetnye Metally, 2019, 12: 29-36.
[40] GORLANOV E S, BAZHIN V Y, FEDOROV S N. Carbide formation at a carbon-graphite lining cathode surface wettable with aluminum [J]. Refractories and Industrial Ceramics, 2016, 57: 292-296.
[41] FENG H K, YU S R, LI Y L, GONG L Y. Effect of ultrasonic treatment on microstructures of hypereutectic Al-Si alloy [J]. Journal of Materials Processing Technology, 2008, 208: 330-350.
[42] ABDELAZIZ M H, SAMUEL A M, DOTY H W, VALTIERRA S, SAMUEL F H. Effect of additives on the microstructure and tensile properties of Al-Si alloys [J]. Journal of Materials Research and Technology, 2019, 8: 2255-2268.
[43] JEON J H, SHIN J H, BAE D H. Si phase modification on the elevated temperature mechanical properties of Al-Si hypereutectic alloys [J]. Materials Science and Engineering A, 2019, 748: 367-370.
[44] LIN Chong, WU Shu-sen, ZHONG Gu, WAN Li, AN Ping. Effect of ultrasonic vibration on Fe-containing intermetallic compounds of hypereutectic Al-Si alloys with high Fe content [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1245-1252.
M. P. KUZ′MIN1, M. Yu. KUZ′MINA1, P. B. KUZ′MIN2
1. Irkutsk National Research Technical University, 83 Lermontov St., Irkutsk, Russia;
2. Limited Liability Company “United Company RUSAL Engineering and Technology Center”, 4 Industrial St., Shelekhov, Irkutsk Region, Russia
摘 要:本工作的主要目的是研究Al(l)-SiO2界面的复杂物理化学过程,开发一种利用由硅生产废料得到的无定形微硅粉生产铸造硅铝合金的新技术。开发使用无定形微硅粉生产亚共晶、共晶和过共晶硅铝合金的有效方法。将预热的无定形微硅粉用氩气流吹入铝熔体(t=900 °C),并进行剧烈混合,得到硅含量为7 wt.%的硅铝合金。先将含硅混合物(60% SiO2,40%Al + 20%3NaF·2AlF3)进行预烧结,使无定形微硅粉还原成结晶硅;再将预烧结产物进行感应熔炼,制备硅含量为21 wt.%的硅铝合金。已经证实,在压片炉料烧结过程中形成的结晶硅可以顺利被铝熔体吸收。氧化还原反应生成的氧化铝溶解在冰晶石中,铝和硅则融合并进入(合金)熔体中。使用无定形微硅粉生产硅铝合金的经济效率计算表明,该项目投资回收期短、盈利能力强。
关键词:铝;硅;硅铝合金;微硅粉;二氧化硅
(Edited by Wei-ping CHEN)
Corresponding author: M. P. KUZ′MIN; Tel/Fax: +79-148858387; E-mail: mike12008@yandex.ru
DOI: 10.1016/S1003-6326(20)65306-7

