


Trans. Nonferrous Met. Soc. China 22(2012) 2523-2528
Preparation of uniform flower-like CuO and flower-like CuO/graphene composite and their application in lithium ion batteries
CHEN Han, FENG Fan, HU Zhong-liang, LIU Fu-sheng, GONG Wen-qiang, XIANG Kai-xiong
College of Metallurgy Engineering, Hunan University of Technology, Zhuzhou 412000, China
Received 9 July 2012; accepted 9 August 2012
Abstract: Flower-like CuO and flower-like CuO/graphene composite were prepared successfully by hydrothermal method. They were characterized by X-ray diffraction, scanning electron microscopy, nitrogen adsorption, temperature-programmed reduction, and thermogravimetric analysis. It is found that the flower-like CuO microspheres, which are composed of CuO nanosheets, possess an average diameter of 4.2 μm and a Brunauer–Emmett–Teller surface area of 12.6 m2/g. Compared with the flower-like CuO, the obtained flower-like CuO/graphene composite shows an enhanced electrochemical performance with a higher capacity of 603 mA·h/g at 0.1 C rate and 382 mA·h/g at 1 C rate, and exhibits a better cycle stability with a high capacity retention of 95.5 % after 50 cycles even though at 1 C rate.
Key words: lithium ion batteries; anode materials; CuO microsphere; graphene
1 Introduction
Li-ion batteries are currently the dominant power sources in mobile phones, notebook computers, and electric vehicles.[1] Transition metal oxides (MO, M=Co, Ni, Cu, Fe) have attracted increasing attention because of the advantages of high reversible capacities [2]. However, they often exhibit severe capacity fading upon cycling mainly due to the large volume change during the charge-discharge cycling. Introducing carbon is a very effective way to overcome these problems. Carbon can buffer the volume effect of active particles, and alleviate their pulverization, and keep good electrical contacts among the active particles [3,4]. These are very beneficial to the electrochemical properties of the transition-metal oxides.
Copper oxide (CuO), a p-type semiconductor with a narrow band gap of about 1.2 eV, has been exploited for Li-ion electrode materials [5]. As an anode material, a conversion mechanism for CuO is expected to be CuO+2Li+?Li2O+Cu-2e. Based on the maximum uptake of 2Li per CuO, the theoretical capacity for CuO is calculated to be 674 mA·h/g, which is much higher than that of conventional graphite anode materials (372 mA·h/g). The copper oxide among transition-metal oxides is of particular interest for its large theoretical capacity, high safety, inexpensiveness and nontoxicity. In recent years, CuO materials with various nano/ microstructures such as nanoparticles, nanowires, [6] nanoribbons, [7] nanorods, [8] nanosheets, [9] pricky, [10] urchin, [11] flower, [12] dumbbell, [13] honeycomb, [14] plate, [15] hollow, [5] and core-shell structures [16] have been successfully synthesized. It has been reported that CuO microspheres show an excellent performance in lithium storage owing to their unique morphology [17].
Herein, we demonstrate a facile template-free method to fabricate flower-like CuO and flower-like CuO/graphene composite by hydrothermal method. Porous structure and graphene networks are constructed by adding graphene to the flower-like CuO microspheres, which play an important role on electrochemical properties, conductivity of composite and volume effect.
2 Experimental
2.1 Material synthesis
In a typical synthesis, 1.0 g of copper nitrate (Cu(NO3)2·H2O, A.R., Sinopharm Chemical Reagent Co., Ltd) or 1.0 g of copper nitrate and 0.03 g graphene (ultrasound treatment for 2 h) were first dissolved in 50.0 mL of absolute alcohol (CH3CH2OH, A.R., Sinopharm Chemical Reagent Co., Ltd) to form a solution in a beaker, and then 30 mL of ammonia water (25%) (NH3·H2O, A.R., Sinopharm Chemical Reagent Co., Ltd), 10 mL of 1 mol/L sodium hydroxide (NaOH, A.R., Sinopharm Chemical Reagent Co., Ltd), and 2.0 g of sodium nitrate (NaNO3, A.R., Sinopharm Chemical Reagent Co., Ltd), were added respectively. After stirring for 10 min to form a clear solution, the resulting solution was transferred into 100 mL stainless-steel autoclave lined with poly(tetrafluoroethylene) (PTFE, Telfon). The autoclave was sealed and maintained at 130 ℃ for 18 h, and then cooled down to room temperature. The resulting precipitate solid was collected by centrifugation, washed with distilled water and absolute ethanol, and finally dried in vacuum at 60 ℃ for 8 h.
2.2 Characterization of materials
X-ray diffraction patterns (XRD) were recorded on a PANalytica X'Pert PRO MPD using the Kα radiation of Cu (λ=1.5418 ?). The pore nature of the samples was investigated using physical adsorption of nitrogen at the liquid-nitrogen temperature (–196 ℃) on an automatic volumetric sorption analyzer (NOVA3200e, Quantachrome). The specific surface areas were determined according to the Brunauer–Emmett–Teller (BET) method in the relative pressure range of 0.05-0.20. The microscopic feature of the as-synthesized powders was characterized by field-emission scanning electron microscopy (SEM) (JSM-6700F, JEOL, Tokyo, Japan) and high-resolution transmission electron microscopy (TEM) (JEM-2010F, JEOL, Tokyo, Japan).
2.3 Electrochemical measurement
The working electrodes were prepared by mixing active materials, acetylene black, and polyvinylidene fluoride (PVDF) with a mass ratio of 75:15:10, using N-methylpyrrolidone (NMP) as a solvent. The resulting slurries were cast onto copper current collectors, then dried at 120 ℃ under vacuum for 24 h. The foils were rolled into thin sheets of 30 μm, then cut into disks with 14 mm in diameter. CR2016 coin-type cells were assembled in an argon-filled glove box, lithium foils were used as counter electrodes, and polypropylene microporous films (Celgard 2400) were used as separators. The liquid electrolyte was 1 mol/L LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1:1, V/V). The galvanostatic charge and discharge tests were carried out by CT2001A LAND testing instrument in a potential range of 0.01-3.0 V at various current rates (1C=674 mA/g). Cyclic voltammogram (CV) was obtained in the voltage of 0-3 V at a scanning rate of 0.1 mV/s at room temperature. Electrochemical impedance spectroscopy (EIS) measurements were conducted using a CHI660D potentiostat over a frequency range from 100 kHz to 10 mHz with an AC oscillation of 5 mV.
3 Results and discussion
3.1 Structure analysis of materials
The flower-like CuO microspheres were successfully synthesized. The reactions involved in the formation of flower-like CuO microspheres are shown in Eqs. (1)-(3).
Cu2++4NH3·H2O→[Cu(NH3)4]2++4H2O (1)
[Cu(NH3)4]2+ +2OH-+2 Na+ →
Cu(OH)2↓+4NH3↑+2Na+ (2)
Cu(OH)2→CuO↓+H2O (3)
[Cu(NH3)4]2+ is first created from copper nitrate and ammonia water[11]. The [Cu(NH3)4]2+ species then react with sodium hydroxide at a certain temperature to form Cu(OH)2[10]. Finally, CuO is formed via the dehydration of Cu(OH)2 according to the chemical reaction given in Eq. (3).
Figure 1(a) shows the XRD patterns of the samples, indicating the formation of pure cubic symmetric CuO (JCPDS No.065—2309). The XRD pattern of as-prepared flower-like CuO/graphene composite shows an additional broad weak peak at 25° attributed to the (002) plane of graphene. Furthermore, Fig. 1(b) shows that the flower-like CuO/graphene composite is composed of 91% CuO and 9% CuO graphene.
3.2 Morphology characterization of materials
Figure 2 shows the SEM and TEM images of flower-like CuO microspheres and flower-like CuO/graphene composites. Figure 2(a) reveals that the flower-like CuO microspheres is very uniform with a size of 3-5 μm. These microspheres are constructed with nanosheets of 50-100 nm, as shown in Fig. 2(b). Figure 2(e) shows the TEM image of flower-like CuO with a dense internal structure. The grain size and morphology are good agreement with Figs. 2(a) and (b). TEM image (Fig. 2(f)) also reveals that the flower-like CuO microspheres are assembled. Figure 2(g) shows a HRTEM image and fast fourier transform (FFT) pattern (inset in Fig. 2(g)) on the edge of nanosheets. The HRTEM image shows a lattice plane distance of 0.25 nm, which is in agreement with the  plane distance of monoclinic CuO. The FFT pattern can be indexed to be the [001] zone axis of monoclinic phase CuO, which is consistent with XRD result and demonstrates that the flower-like CuO microspheres have a single crystalline nature. When graphene was added, Fig. 2(c) shows that the flower-like CuO microspheres and graphene are uniformly dispersed. As shown in Fig. 2(d), the flower-like CuO microspheres are also constructed with nanosheets with a size of 50-100 nm, but become small because the graphene hinders the growth of the flower-like CuO microspheres.
plane distance of monoclinic CuO. The FFT pattern can be indexed to be the [001] zone axis of monoclinic phase CuO, which is consistent with XRD result and demonstrates that the flower-like CuO microspheres have a single crystalline nature. When graphene was added, Fig. 2(c) shows that the flower-like CuO microspheres and graphene are uniformly dispersed. As shown in Fig. 2(d), the flower-like CuO microspheres are also constructed with nanosheets with a size of 50-100 nm, but become small because the graphene hinders the growth of the flower-like CuO microspheres.
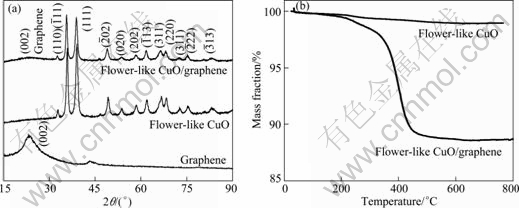
Fig. 1 XRD patterns (a) and TG curves (b) of samples

Fig. 2 SEM images of synthesized samples flower-like CuO (a, b), flower-like CuO/graphene composite (c, d), TEM images (e) of flower-like CuO microspheres and on their edge (f) and HRTEM image (g) (FFT pattern corresponding to single flower-like CuO microspheres is inserted in (g))
3.3 Hole structure of materials
Figure 3(a) shows the N2 adsorption/desorption isotherms of the flower-like CuO microspheres and flower-like CuO/graphene composites. These isotherms with a hysteresis loop in the relative pressure range of 0.7-1.0 belong to the type-IV. The BET surface area is 12.6 m2/g for flower-like CuO microspheres, and 18.7 m2/g for flower-like CuO/graphene composites. The PSD curves in the inset of Fig. 3(a) show that the pore size is larger than 50 nm, beyond the mesoporous level. Figure 3(b) shows the H2-TPR curves of all samples. The maximum position of H2 consumption peak for flower-like CuO/graphene composites is located slightly lower than that for flower-like CuO microspheres, which indicates that the grain size of flower-like CuO microspheres in the composites is smaller that of free-graphene flower-like CuO microspheres, consistent with the SEM and BET results. This may be because flower-like CuO/graphene composites with more exposed surface area are relatively easy to be reduced.
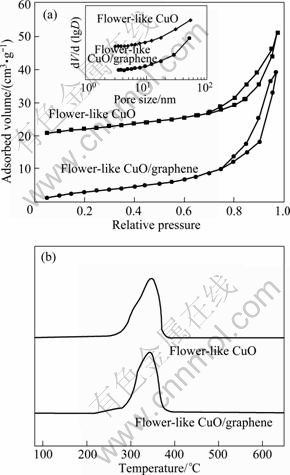
Fig. 3 N2 adsorption-desorption isotherms (a) and H2-TPR (b) curves of samples (BJH-PSD curves of samples are inserted in (a)
3.4 Electrochemical performance of material
Figure 4 shows the electrochemical properties of the flower-like CuO and flower-like CuO/graphene composite. In Fig. 4(a), the initial discharge capacity is 1081 mA·h/g for the flower-like CuO and 967 mA·h/g for the flower-like CuO/graphene composite, and the initial charge capacity is 382 mA·h/g for the flower-like CuO and 603 mA·h/g for the flower-like CuO/graphene composite respectively. Thus, the initial coulomb efficiency of the flower-like CuO/graphene composite (62.4%) is higher than that of the flower-like CuO (35.3%). Their capacity loss in the first cycle is ascribed to diverse irreversible processes such as the inevitable formation of solid electrolyte interface (SEI layer) and electrolyte decomposition. The cycle stability and rate capability are shown in Fig. 4(b). The flower-like CuO/graphene composite exhibits more excellent cycle stability than the flower-like CuO at any rate. The capacity retention of flower-like CuO/graphene attains 95.5% at 1C rate after 50 cycles, but that of flower-like CuO is only 58.5 %. The capacity of flower-like CuO/graphene composite is twice that of the flower-like CuO at any rate. At 0.1C and 0.5C rates, the capacities of the flower-like CuO are respectively 383, 293 and 176 mA·h/g, and the capacities of flower-like CuO/graphene are respectively 603, 455 and 382 mA·h/g. With the rate increasing from 0.1C to 1C, the capacity of flower-like CuO/graphene decreases by only 19.9%, which is much lower than that of flower-like CuO (54.1%).
Figure 4(c) shows the CV curves in the first cycle of the flower-like CuO and flower-like CuO/graphene composite at a scan rate of 0.1 mV/s. In the reduction process, the flower-like CuO and flower-like CuO/graphene composite display four cathodic peaks located at 1.63 V, 0.88 V and 0.72 V and below 0.2 V. These reduction peaks correspond to a multi-step electrochemical reaction due to the creation of a solid solution with a CuO phase, the formation of Cu2O phase, the decomposition of Cu2O into Cu and Li2O and the formation of SEI film on the surface of electrode [18]. In the oxidation process, we can observe three corresponding anodic peaks located at 2.2 V, 1.58 V and below 0.2 V. The strength of cathodic peaks for flower-like CuO/graphene composite is weaker than that for the flower-like CuO, but the strength of anodic peaks for flower-like CuO/graphene composite is stronger than that for the flower-like CuO, indicating that the flower-like CuO/graphene composite exhibits a higher coulomb efficiency than the flower-like CuO. The results of CV curves can be in good agreement with those of galvanostatic charge and discharge. Figure 4(d) shows the Nyquist plots of the flower-like CuO and flower-like CuO/graphene composite. It is well known that the impedance spectra consist of two semicircles and a straight line. The semicircle in the high frequency range corresponds to the resistance of the Li+ ion transfer through the solid electrolyte interphase (SEI) layers, the semicircle in the middle high frequency range is ascribed to the resistance for charge transfer at the electrode/electrolyte interface and the straight line in a low frequency range is the Li+ ion Warburg diffusion resistance in the solid electrode material [19]. The flower-like CuO/graphene composite exhibits smaller semicircles compared with the flower-like CuO, indicating a lower charge transfer resistance within flower-like CuO/graphene composite electrode.

Fig. 4 Initial charge and discharge curves of flower-like CuO and flower-like CuO/graphene composite CuO between 3 and 0.01 V at current rate of 0.1C (67 mA/g) (a), and their cycling performance (b), their CV curves between 0 and 3 V at scanning rate of 0.1 mV/s (c) and their Nyquist plots (d)
The flower-like CuO/graphene composite with an excellent electrochemical performance can be attributed to the porous morphology, which provides a large specific surface area for the intercalation of lithium ions and contains many structural defects to accommodate lithium ions, and graphene networks among flower-like CuO microspheres, which can improve the conductivity of CuO microspheres and accommodate the volume changes for CuO microspheres in charge and discharge processes [17].
4 Conclusions
1) Flower-like CuO and flower-like CuO/graphene composite were prepared by hydrothermal method. The flower-like CuO microspheres possess an average diameter of 4.2 μm and a BET surface area of 12.6 m2/g. The flower-like CuO microspheres are composed of CuO nanosheets.
2) Compared with the flower-like CuO, the obtained flower-like CuO/graphene composite shows an enhanced electrochemical performance with a higher capacity of 603 mA·h/g at 0.1C rate and 382 mA·h/g at 1C rate, and exhibits a better cycle stability with a high capacity retention of 95.5 % after 50 cycles even though at 1C rate.
3) The flower-like CuO/graphene composite possesses an excellent porous structure, which can absorb more electrolytes and shorten the diffusion distance for lithium ions and graphene networks, which can improve its electronic conductivity and buffer the volume effect in charge and discharge processes. Therefore, the flower-like CuO/graphene composite is a potential anode material in Li-ion batteries.
References
[1] ZHAO Yu-qian, JIANG Qing-lai, WANG Wei-gang, DU Ke, HU Guo-rong. Effect of electrolytic MnO2 pretreatment on performance of as-prepared LiMn2O4 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1146-1150.
[2] XIANG J Y, TU J P, ZHANG L, ZHOU Y, WANG X L, SHI S J. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries [J]. Journal of Power Sources, 2010, 195(1): 313-319.
[3] HE Y, HUANG L, CAI J S, ZHENG X M, SUN S G. Structure and electrochemical performance of nanostructured Fe3O4/carbon nanotube composites as anodes for lithium ion batteries [J]. Electrochimica Acta, 2010, 55(3): 1140-1144.
[4] XIANG J Y, TU J P, ZHANG J, ZHONG J, ZHANG D, CHENG J P. Incorporation of MWCNTs into leaf-like CuO nanoplates for superior reversible Li-ion storage [J]. Electrochemistry Communications, 2010, 12(8): 1103-1107.
[5] GAO S Y, YANG S X, SHU J, ZHANG S X, LI Z D, JIANG K. Green fabrication of hierarchical CuO hollow micro/ nanostructures and enhanced performance as electrode materials for lithium-ion batteries [J]. The Journal of Physical Chemistry C, 2008, 112(49): 19324-19328.
[6] JIANG X, HERRICKS T, XIA Y. CuO nanowires can be synthesized by heating copper substrates in air [J]. Nano Letters, 2002, 2(12): 1333-1338.
[7] LIU B, ZENG H C. Mesoscale organization of CuO nanoribbons formation of “dandelions” [J]. Journal of the American Chemical Society, 2004, 126(26): 8124-8125.
[8] TOBOONSUNG B, SINGJAI P. Formation of CuO nanorods and their bundles by an electrochemical dissolution and deposition process [J]. Journal Alloys Compounds, 2011, 509(10): 4132-4137.
[9] JANG K S, KIM J D. In situ catalytic activity of CuO nanosheets synthesized from a surfactant-lamellar template [J]. Journal Nanoscince Nanotechnology, 2011, 11(5): 4496-4500.
[10] XU Y, CHEN D, JIAO X. Fabrication of CuO pricky microspheres with tunable size by a simple solution route [J]. The Journal of Physical Chemistry B, 2005, 109(28): 13561-13566.
[11] XU L, SITHAMBARAM S, ZHANG Y, CHEN C H, JIN L, JOESTEN R, SUIB S L. Novel urchin-like CuO synthesized by a facile reflux method with efficient olefin epoxidation catalytic performance [J]. Chemistry of Materials, 2009, 21(7): 1253-1259.
[12] LIU Y, CHU Y, ZHUO Y J, LI M Y, LI L L, DONG L H. Anion-controlled construction of CuO honeycombs and flowerlike assemblies on copper foils [J]. Crystal Growth and Design, 2007, 7(3): 467-470.
[13] WANG H, SHEN Q, LI X, LIU F. Fabrication of copper oxide dumbbell-like architectures via the hydrophobic interaction of adsorbed hydrocarbon chains [J]. Langmuir, 2009, 25(5): 3152-3158.
[14] LIU Y, CHU Y, ZHUO Y, LI M, LI L, DONG L. Anion-controlled construction of CuO honeycombs and flowerlike assemblies on copper foils [J]. Crystal Growth and Design, 2007, 7(3): 467-470.
[15] YANG M, HE J, HU X, YAN C, CHENG Z. CuO nanostructures as quartz crystal microbalance sensing layers for detection of trace hydrogen cyanide gas [J]. Environmental Science and Technology, 2011, 45(14): 6088-6094.
[16] LI J Y, XIONG S, PAN J, QIAN Y. Hydrothermal synthesis and electrochemical properties of urchin-Like core-shell copper oxide nanostructures [J]. The Journal of Physical Chemistry C, 2010, 114(21): 9645-9650.
[17] FENG J K, XIA H, LAI M O, LU L. Electrochemical performance of CuO nanocrystal film fabricated by room temperature sputtering [J]. Materials Research Bulletin, 2011, 46(3): 424-427.
[18] WANG Z, SU F, MADHAVI S, LOU X W. CuO nanostructures supported on Cu substrate as integrated electrodes for highly reversible lithium storage [J]. Nanoscale, 2011, 3(4): 1618-1623.
[19] REDDY M V, MADHAVI S, RAO G V S, CHOWDARI B V R. Metal oxyfluorides TiOF2 and NbO2F as anodes for Li-ion batteries [J]. Journal of Power Sources, 2006, 162(2): 1312-1321.
花状CuO和花状CuO/石墨烯复合材料的制备及其在锂离子电池中的应用
陈 晗,冯 钒,胡忠良,刘富生,龚文强,向楷雄
湖南工业大学 冶金工程学院,株洲 412000
摘 要:采用水热法合成了花状CuO 和花状CuO/石墨烯复合材料。采用XRD、SEM、TEM、BET、TG对材料的结构、形貌及性能进行表征和分析。花状CuO由CuO的纳米片组成,平均直径为4.2 μm,比表面积为12.6 m2/g。与花状CuO相比,花状CuO/石墨烯复合材料具有更高的充放电容量和更优良的循环稳定性。在0.1C、1C倍率下,其放电容量分别为603 mA·h/g、382 mA·h/g;在1C倍率下,经过50次循环,其容量保持率高达95.5%。
关键词:锂离子电池;负极材料;氧化铜微球;石墨烯
(Edited by LI Xiang-qun)
Foundation item: Project (20110490594) supported by China Postdoctoral Science Foundation
Corresponding author: CHEN Han; Tel: +86-731-22183465; E-mail: lzdxn@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)61495-7