Synthesis and characterization of Na0.44MnO2 nanorods/graphene composite as cathode materials for sodium-ion batteries
来源期刊:中南大学学报(英文版)2019年第6期
论文作者:刘黎 张月 欧阳琰 夏靖 聂苏 刘稳 王先友
文章页码:1510 - 1520
Key words:manganese-based compounds; hydrothermal method; sodium-ion batteries; composite materials
Abstract: Na0.44MnO2 nanorods have been prepared by a hydrothermal method. The experimental parameters have been systematically investigated and optimized. The results show that Na0.44MnO2 nanorods obtained via the hydrothermal treatment at 200 °C for 16 h show the best electrochemical properties, which deliver the high initial discharge capacity of 110.7 mA·h/g at 50 mA/g in potential window 2.0-4.0 V. To further improve their electrochemical properties, a ball milling process with graphene has been carried out to obtain Na0.44MnO2/graphene composite. The initial discharge capacity of Na0.44MnO2/graphene composite is 106.9 mA·h/g at a current density of 50 mA/g. After 100 cycles, the residual discharge capacity is 91.8 mA·h/g and the capacity retention rate is 85.9%, which is much higher than that of pristine Na0.44MnO2 nanorods (74.7%) at the same condition. What is more, when the current density reaches 500 and 1000 mA/g, the corresponding discharge capacities of Na0.44MnO2/graphene composite are about 89 and 78 mA·h/g, respectively, indicating outstanding rate capability.
Cite this article as: ZHANG Yue, OUYANG Yan, LIU Li, XIA Jing, NIE Su, LIU Wen, WANG Xian-you. Synthesis and characterization of Na0.44MnO2 nanorods/graphene composite as cathode materials for sodium-ion batteries [J]. Journal of Central South University, 2019, 26(6): 1510-1520. DOI: https://doi.org/10.1007/s11771-019-4107-6.

ARTICLE
J. Cent. South Univ. (2019) 26: 1510-1520
DOI: https://doi.org/10.1007/s11771-019-4107-6
ZHANG Yue(张月)1, OUYANG Yan(欧阳琰)1, LIU Li(刘黎)1, 2, XIA Jing(夏靖)1,
NIE Su(聂苏)1, LIU Wen(刘稳)1, WANG Xian-you(王先友)1
1. National Base for International Science & Technology Cooperation, National Local Joint Engineering
Laboratory for Key Materials of New Energy Storage Battery, Hunan Province Key Laboratory of
Electrochemical Energy Storage and Conversion, Key Laboratory of Environmentally Friendly
Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University,Xiangtan 411105, China;
2. Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University, Tianjin 300071, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: Na0.44MnO2 nanorods have been prepared by a hydrothermal method. The experimental parameters have been systematically investigated and optimized. The results show that Na0.44MnO2 nanorods obtained via the hydrothermal treatment at 200 °C for 16 h show the best electrochemical properties, which deliver the high initial discharge capacity of 110.7 mA·h/g at 50 mA/g in potential window 2.0-4.0 V. To further improve their electrochemical properties, a ball milling process with graphene has been carried out to obtain Na0.44MnO2/graphene composite. The initial discharge capacity of Na0.44MnO2/graphene composite is 106.9 mA·h/g at a current density of 50 mA/g. After 100 cycles, the residual discharge capacity is 91.8 mA·h/g and the capacity retention rate is 85.9%, which is much higher than that of pristine Na0.44MnO2 nanorods (74.7%) at the same condition. What is more, when the current density reaches 500 and 1000 mA/g, the corresponding discharge capacities of Na0.44MnO2/graphene composite are about 89 and 78 mA·h/g, respectively, indicating outstanding rate capability.
Key words: manganese-based compounds; hydrothermal method; sodium-ion batteries; composite materials
Cite this article as: ZHANG Yue, OUYANG Yan, LIU Li, XIA Jing, NIE Su, LIU Wen, WANG Xian-you. Synthesis and characterization of Na0.44MnO2 nanorods/graphene composite as cathode materials for sodium-ion batteries [J]. Journal of Central South University, 2019, 26(6): 1510-1520. DOI: https://doi.org/10.1007/s11771-019-4107-6.
1 Introduction
Energy and environmental issues have become the most concerned issues in the world nowadays, and people’s growing needs make energy issues more and more serious. So it is crucial to apply alternative new energy systems to realize society sustainable development [1], which brings about the urgent need of efficient devices. Lithium-ion batteries have been widely applied in various portable energy storage devices [2-4]. However, due to the shortage of lithium resources and the high price, sodium-ion batteries are becoming more and more attractive, whose energy storage mechanism is similar to lithium ion batteries but the price is much lower due to the abundant sodium resources [5-10].ZHANG Yue and OUYANG Yan contributed equally to this work.
Recently, cathode materials of sodium ion batteries mainly focused on layered and tunnel type transition metal oxides/sulfides/fluorides, polyanionic compounds, Prussian blue and its derivatives, and organic compounds [11, 12]. However, due to the larger radius of Na+, cathode material structure of sodium ion battery is more likely to collapse in the sodium ions insertion/ extraction processes [13, 14]. Manganese-based cathode materials (NaxMnO2, 0
In this work, we prepared Na0.44MnO2 nanorods by a hydrothermal method and optimized the experimental parameters of hydrothermal process. Moreover, the as-prepared Na0.44MnO2 nanorods were ball-milled with graphene to form Na0.44MnO2/graphene composite, which shows much better electrochemical performance than that of pristine Na0.44MnO2 nanorods.
2 Experimental
2.1 Materials preparation
Firstly, 6.0 g NaOH was dissolved in 40 mL deionized water and magnetically stirred for 10 min. Then, adding 0.79 g KMnO4 to the above solution with continous stirring to form 50 mL purple mixed solution. Finally, 50 mL deionized water containing 2.37 g MnSO4·H2O was added to purple mixed solution under the magnetic stirring. The brown precipitate was rapidly formed and the suspension was aged at room temperature for 24 h to wash, filter, and dry under vacuum at 80 °C for 12 h to obtain a tan solid of Na-birnsssite precursor.
To prepare Na0.44MnO2 nanorods, 0.2 g Na-birnsssite precursor was added in a polytetrafluoroethylene reactor with 50 mL(15.0 mol/L) NaOH solution. After magnetically stirring for 30 min, it was sealed and placed in drying oven at certain temperature for certain time. After cooling, centrifuging and drying, a brown solid is obtained. Finally, the brown solid was calcined at 600 °C for 2 h in a muffle furnace to get final product of Na0.44MnO2.
The Na0.44MnO2/graphene composite was synthesized by ball-milling the as-prepared Na0.44MnO2 nanorods and commercial graphene (CG) (The Sixth Element Materials Technology Co, Ltd, China) with a mass ratio of 10:1 for 3 h at 400 r/min.
2.2 Materials characterization
The crystal structure of the sample was identified by powder X-ray diffraction (XRD). The XRD data was obtained by a Rigaku D/MAX-2500 power diffractometer. Morphology and internal structure of samples were characterized by the scanning electron microscope (SEM, JEOL JSM-6610) and the transmission electron microscopy (TEM, JEOL JEM-2100F). The distribution of elements in the sample was decided by energy-dispersive X-ray spectrometry (EDS).
2.3 Electrochemical tests
The pristine Na0.44MnO2 nanorods or Na0.44MnO2/graphene composite, acetylene black, and binder (PVDF) were mixed with the mass ratio of 80:10:10 in 1-methyl-2-pyrrolidone (NMP) solution to form mixture slurry. After the slurry is uniformly mixed, it is evenly spread on a current collector of metal aluminum foil by a coater, dried at 60 °C for 24 h, finally cut into disks with diameter of 1 cm as working electrodes. Using a sodium metal plate as a reference electrode, 1 mol/L NaClO4 (V[EC(ethylene carbonate)]: V[PC(propylene carbonate)]=1:1) as the electrolyte and GF/D glass fiber as diaphragm to assemble 2025 coin-type cells in a glove box with high purity argon continuous circulation, charge/discharge test was carried out in a battery test system (BT3008W, Neware, Shenzhen, China) and the cyclic voltammetry (CV) measurements and electrochemical impedance spectroscopy (EIS) tests were performed at CHI660e electrochemical workstation (Chenhua, Shanghai, China).
3 Results and discussion
3.1 Effect of hydrothermal temperature and time
The hydrothermal reaction was firstly carried out with the same reaction time of 16 h at different temperatures (160, 180 and 200 °C) and the XRD patterns of the products are shown in Figure 1(a). When the temperatures are 160 and 180 °C, although main peaks of XRD patterns are corresponding to the Na0.44MnO2, there are also some impurity peaks (marked with  ), which are from Na-birnssite precursor. As the temperature reaches to 200 °C, the impurity peaks disappear as well as the peaks can be well consistent with the standard data files of Na0.44MnO2 (PDF 27-0750). These data indicate that 200 °C is the optimal hydrothermal temperature.
), which are from Na-birnssite precursor. As the temperature reaches to 200 °C, the impurity peaks disappear as well as the peaks can be well consistent with the standard data files of Na0.44MnO2 (PDF 27-0750). These data indicate that 200 °C is the optimal hydrothermal temperature.
In addition, XRD patterns of products with different hydrothermal reaction times (8, 16, and 24 h) at the same hydrothermal temperature of 200 °C are shown in Figure 1(b). Most peaks of products obtained at different hydrothermal time can correspond to Na0.44MnO2 phase. The (040) crystal plane at 13.7° has been marked in curves, which belongs to Na0.44MnO2 phase and is consistent with reported Na0.44MnO2 products [24, 29, 32, 33]. There exists a small impurity peak at 16.2° (marked with ◆), which is assigned to Na2Mn3O7 phase. Although the impurity phase Na2Mn3O7 exists in products, the peak intensity of Na2Mn3O7 is tiny and unconspicuous, which does not have much effect on the primary phase Na0.44MnO2.
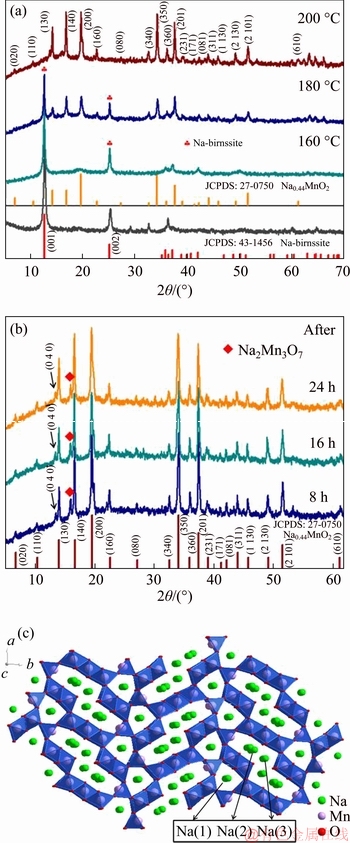
Figure 1 XRD patterns of Na0.44MnO2 nanorods synthesized at different hydrothermal temperatures for 16 h (a) and Na0.44MnO2 nanorods synthesized at hydrothermal temperatures of 200 °C for different hydrothermal time (b), crystal structure diagram of Na0.44MnO2 (c)
3.2 Effect of hydrothermal reaction time on electrochemical performance
The electrochemical performance of three products obtained at hydrothermal temperature of 200 °C for different hydrothermal time (8, 16 and 24 h) is shown in Figure 2. The first charge and discharge curves of the three samples are shown in Figures 2(a)-(c). The obvious charge and discharge platforms correspond to the multiphase transformation processes. The first discharge capacities of the three products (8, 16 and 24 h) are 107.9, 110.7 and 106.3 mA·h/g, respectively. In addition, cycle capability comparison curves at 0.05 A/g are shown in Figure 2(d), and the residual discharge capacities of 8, 16, and 24 h after 100 cycles are 73.3, 82.7 and 82.4 mA·h/g, respectively. The corresponding capacity retention rates are 67.9%, 74.7% and 77.5%, respectively. Among them, the Na0.44MnO2 nanorods synthesized by hydrothermal reaction for 16 h own the highest discharge capacities. For further comparison of these three products, the cycle capability at 0.5 A/g is shown in Figure 2(e), the capacity retentions after 50 cycles for the products obtained by hydrothermal reaction for 8, 16, and 24 h are 65.5%, 76.2% and 76.0%, respectively. It is noted that although the product obtained through the hydrothermal treatment of 16 h shows slightly less capacity retention than that of the product obtained for 24 h at 0.05 A/g, its specific capacity is much higher at high current density.
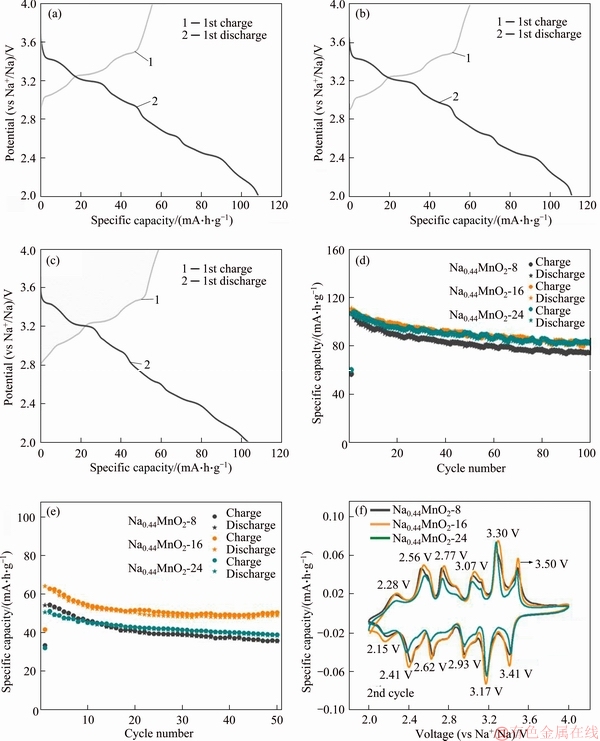
Figure 2 Initial charge/discharge curves of products obtained at 0.05 A/g and 0.42C for hydrothermal time of 8 h (a),16 h (b), and 24 h (c) and cycling behaviors at 0.05 A/g (d) and 0.5 A/g for three products (e), and CV curves at a scanning rate of 0.1 mV/s for three products (f)
The CV curves (Figure 2(f)) of the three products at the second cycle show similar characteristics, which have at least 6 pairs of redox peaks derived from complex multiphase transition during charge/discharge processes and are similar to the reported manganese-based cathode material [34, 35]. The positions of six pairs of redox peaks for all samples approximately locate at 2.15 V/2.28 V,2.41 V/2.56 V, 2.62 V/2.77 V, 2.93 V/3.07V, 3.17 V/3.30 V and 3.41 V/3.50 V, respectively, and these locations of peaks are well corresponding to charge/discharge platforms in Figures 2(a)-(c). The Na0.44MnO2 synthesized by hydrothermal treatment of 16 h owns the highest peaks intensity and the largest peak area, indicating that this product has the highest specific capacity, which is well consistent with charge-discharge properties shown in Figures 2(a)-(e).
The above discussions indicate that Na0.44MnO2 synthesized by hydrothermal treatment at 200 °C for 16 h shows the best sodium storage performance.
3.3 Characterizations of Na0.44MnO2 obtained at optimal hydrothermal parameters
In order to further study the morphology and micro structure of Na0.44MnO2obtained at optimal hydrothermal condition, which is hydrothermal treatment at 200 °C for 16 h, SEM, TEM HRTEM and EDS were carried out. As shown in SEM images (Figures 3(a) and (b)) and TEM image (Figure 3(c)), the sample exhibits a relatively uniform nanorods structure with an average diameter between 100 and 200 nm and a length of about 2 to 5 μm. In the HRTEM diagram (Figure 3(d)), the lattice spacing of 0.39 nm can be observed, corresponding to the (160) crystal plane of Na0.44MnO2 nanorods. The inset (in Figure 3(d)) is the corresponding selected area electron diffraction pattern (SAED) image and it can be seen that some diffraction spots are corresponding to crystal planes (160), (340), (350), (081), (1130) and (2130) of Na0.44MnO2 nanorods. It can be seen from EDS mapping images (Figure 3(e)) and EDS spectra (Figure 3(f)) of the sample that the sample only contains three elements of Na, Mn and O and the distribution of these elements are uniform in the sample. The elements content of Na and Mn are shown in the table of Figure 3(f) and the molar ratio of Na: Mn is 0.4397, which is well close to the elements ratio in Na0.44MnO2.
3.4 Characterizations of Na0.44MnO2/graphene composite
Figure 4(a) shows the XRD patterns of the pristine Na0.44MnO2 and the Na0.44MnO2/graphene composite. The diffraction peaks of the two samples are well consistent with Na0.44MnO2 (JCPDS standard card number: 27-0750, space group:Pbam) and the diffraction peaks are sharp. In addition, compared with pristine Na0.44MnO2, the diffraction peaks of Na0.44MnO2/graphene composite do not change significantly, indicating that the addition of graphene does not affect the crystal structure of pristine Na0.44MnO2.
The SEM and TEM images of Na0.44MnO2/ graphene composite are shown in Figures 4(b) and (c). Na0.44MnO2 exhibits a uniform and well- crystallized nanorods-like structure with a diameter of 100-200 nm. The graphene sheets are dispersed around the Na0.44MnO2 nanorods to form a uniform dense conductive network. In HRTEM (Figure 4(d)), the lattice fringe spacing is 0.45 nm, corresponding to the (200) crystal plane of the Na0.44MnO2 nanorods. The amorphous stripes belong to graphene. Graphene has thin thickness and large specific surface area, which can shorten the ions diffusion path and improve the electrical conductivity of the Na0.44MnO2 nanorods. Therefore, the electrochemical performance of Na0.44MnO2 nanorods could be expected to be improved by compositing with graphene.
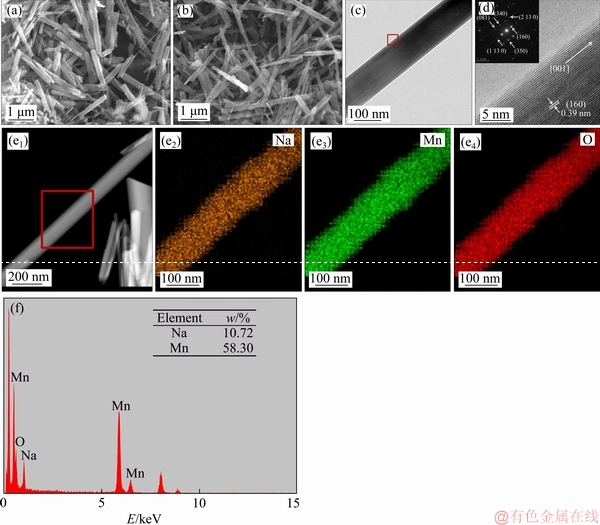
Figure 3 SEM images (a, b), TEM image (c), corresponding HRTEM and SAED image (d), EDS mapping images (e) and EDS spectrum (f) of Na0.44MnO2 prepared via hydrothermal treatment at 200 °C for 16 h
3.5 Electrochemical performance of Na0.44MnO2/ graphene composite
The CV comparison curves of the second cycle of pristine Na0.44MnO2 and Na0.44MnO2/graphene composite at 0.1 mV/s are shown in Figure 5(a). The approximate positions of peaks pairs have been labeled in curves. The potential difference between each pair of redox peaks is reduced for the composite, indicating that the polarization of Na0.44MnO2/graphene composite is smaller than that of pristine Na0.44MnO2 during charge and discharge processes. Therefore, the addition of graphene improved the electrochemical reversibility of Na0.44MnO2. The CV curves (Figure 5(b)) of the first three cycles of Na0.44MnO2/graphene show good coincidence, revealing that the crystal structure of Na0.44MnO2/graphene composite is relatively stable and the composite has good reversibility during sodium ions insertion/ deinsertion processes.
The comparison of charge-discharge properties for pristine Na0.44MnO2 and Na0.44MnO2/graphene composite is shown in Figure 6. Figure 6(a) shows the cycle performance curves of two samples at 50 mA/g. The Na0.44MnO2/graphene composite delivers an initial discharge capacity of 106.9 mA·h/g at 50 mA/g, which is slightly lower than that of pristine Na0.44MnO2. The residual discharge capacity of the Na0.44MnO2/graphene composite after 100 cycles is 91.8 mA·h/g and the capacity retention is 85.9%, which are much higher than those of pristine Na0.44MnO2 (74.7%). The rate performance curves are shown in Figure 6(b), at the current densities of 50, 100, 500 and 1000 mA/g, the discharge capacities of Na0.44MnO2 are about 111, 99, 66 and 39 mA·h/g, respectively, and the discharge capacities of Na0.44MnO2/graphene composite are about 108, 100, 89 and 78 mA·h/g, respectively. Na0.44MnO2/graphene composite shows much better rate capability than that of pristine Na0.44MnO2. From the corresponding charge/discharge curves of pristine Na0.44MnO2 (Figure 6(c)) and Na0.44MnO2/ graphene composite (Figure 6(d)), it is more intuitive to see that the Na0.44MnO2/graphene composite has better rate performance. The obvious charge and discharge platforms well correspond to the redox peak pairs of the CV curves, indicating the complex sodium ions insertion and extraction processes. Graphene is a new type two-dimensional carbon nanomaterial [28, 32, 36-38]. Due to its high specific surface area, high surface activity, high electrical conductivity and high stability, it has become a research hotspot in the field of electrochemical energy storage. After being combined with the Na0.44MnO2 nanorods, graphene can form a uniform dense conductive network around the Na0.44MnO2 nanorods, which is beneficial to the transfer of electrons and improves the conductivity of the Na0.44MnO2 nanorods, thereby improving the electrochemical performance of the Na0.44MnO2 nanorods.
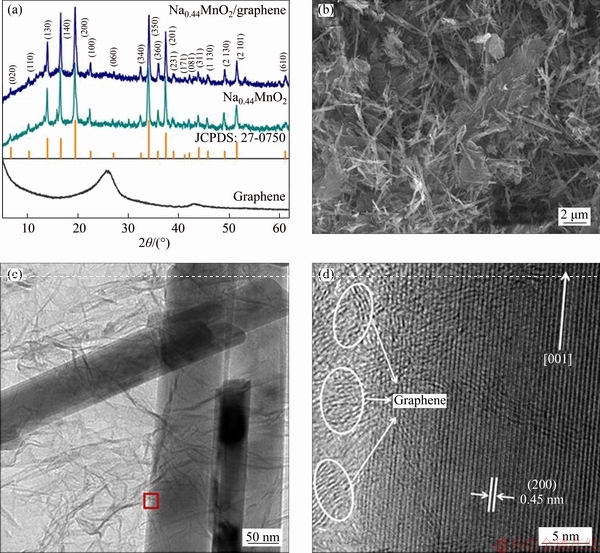
Figure 4 XRD spectra of pristine Na0.44MnO2 nanorods and Na0.44MnO2/graphene composite (a), SEM image (b), TEM (c) and HRTEM (d) images of Na0.44MnO2/graphene composite
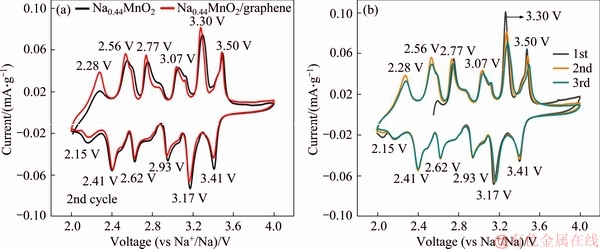
Figure 5 Comparison of CV curves of pristine Na0.44MnO2 and Na0.44MnO2/graphene composite at the second cycle (a) and first three cycles CV curves of Na0.44MnO2/graphene composite (b) (Scan rate of 0.1 mV/s and voltage range of 2.0-4.0 V)
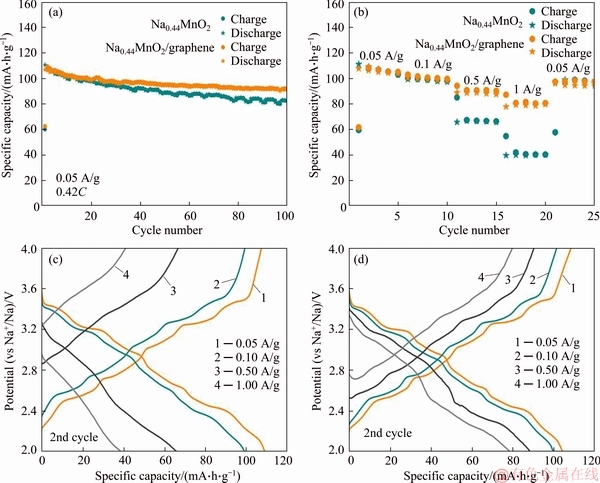
Figure 6 Comparison curves of cycle performance (at 50 mA/g current, voltage range of 2.0-4.0 V) of pristine Na0.44MnO2 and Na0.44MnO2/graphene composite (a), comparison curves of rate capability of two samples (b), charge/discharge curves at different current density for pristine Na0.44MnO2 (c) and Na0.44MnO2/graphene composite (d)
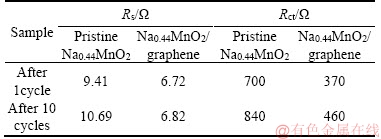
The electrochemical performance for two samples was further studied by electrochemical impedance spectroscopy (EIS) tests, which were carried out on Na0.44MnO2 and Na0.44MnO2/ graphene composite after 1 cycle and 10 cycles. The Nyquist plots of the samples and the simulated equivalent circuit are shown in Figure 7. In general, the semicircle in high-frequency area represents the charge-transfer resistance (Rct) and the rising line is corresponding to ion diffusion [39]. The Rs is related to electrolyte and ohm resistance, which is negligible [40]. The fitted Rct value of 700 Ω and 840 Ω can be got for pristine Na0.44MnO2 after 1 cycle and 10 cycles, respectively, which is much higher than Na0.44MnO2/graphene composite, whose fitted Rct values are 370 Ω and 460 Ω after 1 cycle and 10 cycles, respectively. These data demonstrate that the addition of graphene effectively alleviate the charge transfer resistance and the fitted impedance values are shown in Table 1, where the lower values of Rct and Rs for Na0.44MnO2/graphene composite well prove that the addition of graphene is beneficial to transfer of electrons and enhances the electrochemical performance of Na0.44MnO2.
4 Conclusions
Pristine Na0.44MnO2 nanorods with the highest purity and good sodium storage performance have been obtained through optimation of hydrothermal reaction conditions. In addition, Na0.44MnO2/ graphene composite is synthesized by ball milling pristine Na0.44MnO2 nanorods with graphene, which displays enhanced electrochemical performance. In the potential window 2.0-4.0 V, the initial discharge capacity of Na0.44MnO2/graphene composite is 106.9 mA·h/g at a current density of 50 mA/g. After 100 cycles, the residual specific capacity is 91.8 mA·h/g, and the capacity retention rate is 85.9%. The cycle performance of Na0.44MnO2/graphene is much better than that of pristine Na0.44MnO2. Besides, the composite shows outstanding rate capability. At the current densities of 50, 100, 500 and 1000 mA/g, the discharge capacities of Na0.44MnO2/graphene composites are 108, 100, 89 and 78 mA·h/g, respectively. The addition of graphene effectively improves the electrochemical performance of Na0.44MnO2 nanorods, mainly due to the increased conductivity of the Na0.44MnO2 nanorods.
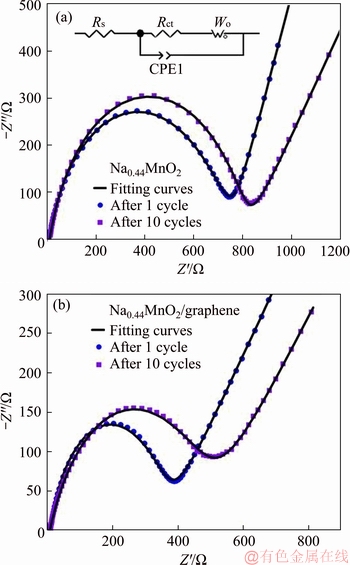
Figure 7 EIS curves of Na0.44MnO2 (a) and Na0.44MnO2/graphene composite (b) after 1 cycle and 10 cycles (Inset shows simulated equivalent circuit model)
Table 1 Rs and Rct values of Na0.44MnO2 and Na0.44MnO2/graphene electrodes after 1 cycle and 10 cycles
References
[1] SLATER M D, KIM D, LEE E, JOHNSON C S. Sodium-ion batteries [J]. Advanced Functional Materials, 2013, 23(8): 947-958.
[2] XIA Jing, LIU Li, XIE Jian-jun, YAN Han-xiao, YUAN Yu-ting, CHEN Man-fang, HUANG Cheng, ZHANG Yue, NIE Su, WANG Xian-you. Layer-by-layered SnS2 /graphene hybrid nanosheets via ball-milling as promising anode materials for lithium ion batteries [J]. Electrochimica Acta, 2018, 269: 452-461.
[3] XIE Jian-jun, PEI Yi, LIU Li, GUO Sheng-ping, XIA Jing, LI Min, OUYANG Yan, ZHANG Xiao-yan, WANG Xian-you. Hydrothermal synthesis of antimony oxychlorides submicron rods as anode materials for lithium-ion batteries and sodium-ion batteries [J]. Electrochimica Acta, 2017, 254: 246-254.
[4] YI Ling-guang, LIU Li, GUO Guo-xiong, CHEN Xiao-ying, ZHANG Yue, YU Shu-yang, WANG Xian-you. Expanded graphite@SnO2@polyaniline composite with enhanced performance as anode materials for lithium ion batteries [J]. Electrochimica Acta, 2017, 240: 63-71.
[5] XIE Jian-jun, LIU Li, XIA Jing, ZHANG Yue, LI Min, OUYANG Yan, NIE Su, WANG Xian-you. Template-free synthesis of Sb2S3 hollow microspheres as anode materials for lithium-ion and sodium-ion batteries [J]. Nano-Micro Letters, 2018, 10(1): 12.
[6] YUAN D D, WANG Y X, CAO Y L, AI X P, YANG H X. Improved electrochemical performance of Fe-substituted NaNi0.5Mn0.5O2 cathode materials for sodium-ion batteries [J]. ACS Applied Materials & Interfaces, 2015, 7(16): 8585-8591.
[7] LI Huang-xu, CHEN Xiao-bin, JIN Ting, BAO Wei-zhai, ZHANG Zhi-an, JIAO Li-fang. Robust graphene layer modified Na2MnP2O7 as a durable high-rate and high energy cathode for Na-ion batteries [J]. Energy Storage Materials, 2019, 16: 383-390.
[8] JIN Ting, HAN Qing-qing, WANG Yi-jing, JIAO Li-fang. 1D nanomaterials: Design, synthesis, and applications in sodium-ion batteries [J]. Small, 2018, 14(2): 1703086.
[9] WANG Xiao-jun, CAO Kang-zhe, WANG Yi-jing, JIAO Li-fang. Controllable N-doped CuCo2O4@C film as a self-supported anode for ultrastable sodium-ion batteries [J]. Small, 2017, 13(29): 1700873.
[10] HE Han-nan, GAN Qing-meng, WANG Hai-yan, XU Gui-liang, ZHANG Xiao-yi, HUANG Dan, FU Fang, TANG You-gen, AMINE K, SHAO Min-hua. Structure-dependent performance of TiO2/C as anode material for Na-ion batteries [J]. Nano Energy, 2018, 44: 217-227.
[11] SUN Yang, GUO Shao-hua, ZHOU Hao-sheng. Exploration of advanced electrode materials for rechargeable sodium-ion batteries [J]. Advanced Energy Materials, 2018: 1800212.
[12] LI Wei-jie, HAN Chao, WANG Wan-lin, GEBERT F, CHOU Shu-lei, LIU Hua-kun, ZHANG Xin-he, DOU Shi-xue. Commercial prospects of existing cathode materials for sodium ion storage [J]. Advanced Energy Materials, 2017, 7(24): 1700274.
[13] JIN Ting, LIU Yong-chang, LI Yang, CAO Kang-zhe, WANG Xiao-jun, JIAO Li-fang. Electrospun NaVPO4F/C nanofibers as self-standing cathode material for ultralong cycle life Na-ion batteries [J]. Advanced Energy Materials, 2017, 7(15): 1700087.
[14] HE Han-na, HUANG Dan, PANG Wei-kong, SUN Dan, WANG Qi, TANG You-gen, JI Xiao-bo, GUO Zai-ping, WANG Hai-yan. Plasma-induced amorphous shell and deep cation-site S doping endow TiO2 with extraordinary sodium storage performance [J]. Advanced Materials, 2018, 30(26): 1801013.
[15] ZHANG Zi-he, WU Di-hua, ZHANG Xu, ZHAO Xu-dong, ZHANG Hai-chang, DING Fei, XIE Zhao-jun, ZHOU Zhen. First-principles computational studies on layered Na2Mn3O7 as a high-rate cathode material for sodium ion batteries [J]. Journal of Materials Chemistry A, 2017, 5(25): 12752- 12756.
[16] ADAMCZYK E, PRALONG V. Na2Mn3O7:A suitable electrode material for Na-ion batteries? [J]. Chemistry of Materials, 2017, 29(11): 4645-4648.
[17] ABAKUMOV A M, TSIRLIN A A, BAKAIMI I, TENDELOO G V, LAPPAS A. Multiple twinning as a structure directing mechanism in layered rock-salt-type oxides: NaMnO2 polymorphism, redox potentials, and magnetism [J]. Chemistry of Materials, 2014, 26(10): 3306-3315.
[18] WANG Qi-di, YANG Wei, KANG Fei-yu, LI Bao-hua. Na2Mn3+0.3Mn4+2.7O6.85: A cathode with simultaneous cationic and anionic redox in Na-ion battery [J]. Energy Storage Materials, 2018, 14: 361-366.
[19] SU Da-wei, WANG Cheng-yin, AHN H, WANG Guo-xiu. Single crystalline Na0.7MnO2 nanoplates as cathode materials for sodium-ion batteries with enhanced performance [J]. Chemistry—A European Journal, 2013, 19(33): 10884- 10889.
[20] SAUVAGE F, LAFFONT L, TARASCON J M, BAUDRIN E. Study of the insertion/deinsertion mechanism of sodium into Na0.44MnO2 [J]. Inorganic Chemistry, 2007, 46(8): 3289-3294.
[21] LIU Sheng, FAN Cheng-zhi, ZHANG Yuan, LI Cheng-hui, YOU Xiao-zeng. Low-temperature synthesis of Na2Mn5O10 for supercapacitor applications [J]. Journal of Power Sources, 2011, 196(23): 10502-10506.
[22] HOU Yan, TANG Hong-wei, LI Bao, CHANG Kun, CHANG Zhao-rong, YUAN Xiao-zi, WANG Hai-jiang. Hexagonal-layered Na0.7MnO2.05 via solvothermal synthesis as an electrode material for aqueous Na-ion supercapacitors [J]. Materials Chemistry and Physics, 2016, 171: 137-144.
[23] BILLAUD J, CL MENT R J, ARMSTRONG A R, CANALES-VAZQUEZ J, ROZIER P, GREY C P, BRUCE P G. β-NaMnO2:A high-performance cathode for sodium-ion batteries [J]. Journal of the American Chemical Society, 2014, 136(49): 17243-17248.
MENT R J, ARMSTRONG A R, CANALES-VAZQUEZ J, ROZIER P, GREY C P, BRUCE P G. β-NaMnO2:A high-performance cathode for sodium-ion batteries [J]. Journal of the American Chemical Society, 2014, 136(49): 17243-17248.
[24] WANG C H, YEH Y W, WONGITTHAROM N, WANG Yi-chen, TSENG C J, LEE S W, CHANG Wen-sheng, CHANG J K. Rechargeable Na/Na0.44MnO2 cells with ionic liquid electrolytes containing various sodium solutes [J]. Journal of Power Sources, 2015, 274: 1016-1023.
[25] YU Jiang-ying, HONG Mao-rong, WANG Li, HUANG Kai, GUO Yu-xian. Synthesis and gas-sensing properties of P-type Na0.44MnO2 nanoribbons [J]. Materials Letters, 2016, 164: 440-443.
[26] LIU Cai, LI Jiang-gang, ZHAO Peng-xiang. Fast preparation of Na0.44MnO2 nanorods via a high NaOH concentration hydrothermal soft chemical reaction and their lithium storage properties [J]. Journal of Nanoparticle Research, 2015, 17(3): 142.
[27] LIU Xiao, ZHANG Ning, NI Jiang-feng, GAO Li-jun. Improved electrochemical performance of sol–gel method prepared Na4Mn9O18 in aqueous hybrid Na-ion supercapacitor [J]. Journal of Solid State Electrochemistry, 2013, 17(7): 1939-1944.
[28] XU Mao-wen, NIU Yu-bin, CHEN Chuan-jun, SONG Jie, BAO Shu-juan. Synthesis and application of ultra-long Na0.44MnO2 submicron slabs as a cathode material for Na-ion batteries [J]. RSC Adv., 2014, 4(72): 38140-38143.
[29] ZHAO Li-wei, NI Jiang-feng, WANG Hai-bao, GAO Li-jun. Na0.44MnO2–CNT electrodes for non-aqueous sodium batteries [J]. RSC Advances, 2013, 3(18): 6650: 6655.
[30] WANG Xu-yang, ZHOU Xu-feng, YAO Ke, ZHANG Jiang-gang, LIU Zhao-ping. A SnO2/graphene composite as a high stability electrode for lithium ion batteries [J]. Carbon, 2011, 49(1): 133-139.
[31] DAVID L, BHANDAVAT R, SINGH G. MoS2/Graphene composite paper for sodium-ion battery electrodes [J]. ACS Nano, 2014, 8(2): 1759-1770.
[32] HE Xin, WANG Jun, QIU Bao, PAILLARD E, MA Chu-ze, CAO Xia, LIU Hao-dong, STAN M C, LIU Hai-dong, GALLASH T, MENG Y S, LI Jie. Durable high-rate capability Na0.44MnO2 cathode material for sodium-ion batteries [J]. Nano Energy, 2016, 27: 602-610.
[33] RUFFO R, FATHI R, KIM D J, JUNG Y H, MARI C M, KIM K. Impedance analysis of Na0.44MnO2 positive electrode for reversible sodium batteries in organic electrolyte [J]. Electrochimica Acta, 2013, 108: 575-582.
[34] KIM H, KIM D J, SEO D H, YEOM M S, KANG K, KIM D K, JUNG Y. Ab initio study of the sodium intercalation and intermediate phases in Na0.44MnO2 for sodium-ion battery [J]. Chemistry of Materials, 2012, 24(6): 1205-1211.
[35] CAO Yu-liang, XIAO Li-fen, WANG Wei, CHOI D, NIE Zi-min, YU Jiang-guo, SARAF L V, YANG Zhen-guo, LIU Jun. Reversible sodium ion insertion in single crystalline manganese oxide nanowires with long cycle life [J]. Advanced Materials, 2011, 23(28): 3155-3160.
[36] LIU Qian-qian, HU Zhe, CHEN Ming-zhe, GU Qin-fen, DOU Yu-hai, SUN Zi-qi, CHOU Shu-lei, DOU Shi-xue. Multiangular rod-shaped Na0.44MnO2 as cathode materials with high rate and long life for sodium-ion batteries [J]. ACS Applied Materials & Interfaces, 2017, 9(4): 3644-3652.
[37] FERRARA C, TEALDI C, DALL’ASTA V, BUCHHOLZ D, CHAGAS L G, QUARTARONE E, BERBENNI V, PASSERINI S. High-performance Na0.44MnO2 slabs for sodium-ion batteries obtained through urea-based solution combustion synthesis [J]. Batteries, 2018, 4(1): 8.
[38] DAI Ke-hua, MAO Jing, SONG Xiang-yun, BATTAGLIA V, LIU Gao. Na0.44MnO2 with very fast sodium diffusion and stable cycling synthesized via polyvinylpyrrolidone- combustion method [J]. Journal of Power Sources, 2015, 285: 161-168.
[39] LUO Chao, LANGROCK A, FAN Xiu-lin, LIANG Yu-jia, WANG Chun-sheng. P2-type transition metal oxides for high performance Na-ion battery cathodes [J]. Journal of Materials Chemistry A, 2017, 5(34): 18214-18220.
[40] WU Fang, ZHANG Xiao-xiao, ZHAO Tao-lin, LI Li, XIE Man, CHEN Rui-jie. Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries [J]. ACS Applied Materials & Interfaces, 2015, 7(6): 3773-3781.
(Edited by FANG Jing-hua)
中文导读
钠离子电池正极复合材料Na0.44MnO2/石墨烯的合成与性能
摘要:本文通过水热法合成了Na0.44MnO2纳米棒,并系统地研究和优化了合成该材料的实验参数。实验结果表明,在200°C 下,水热反应16 h获得的Na0.44MnO2纳米棒展现了最好的电化学性能。在2.0~4.0 V的电压窗口,50 mA/g电流密度下,该材料具有110.7 mA·h/g的初始放电比容量,循环100周后的容量保持率为74.7%。为了进一步提高该材料的电化学性能,将石墨烯与其混合球磨,得到了Na0.44MnO2/石墨烯复合材料。在50 mA/g电流密度下,该复合材料首次放电比容量为106.9 mA·h/g,100周循环后,放电比容量仍保持为91.8 mA·h/g,容量保持率为85.9%。此外,当电流密度提高到500 和1000 mA/g时,该复合材料分别具有89 和 78 mA·h/g 的放电比容量。与石墨烯复合,Na0.44MnO2材料的循环性能与倍率性能得到了显著提高。
关键词:锰基化合物;水热法;钠离子电池;复合材料
Foundation item: Project(51672234) supported by the National Natural Science Foundation of China; Project(1337304) supported by the Program for Innovative Research Cultivation Team in University, Ministry of Education, China
Received date: 2018-10-25; Accepted date: 2019-03-06
Corresponding author: LIU Li, PhD, Professor; Tel: +86-15080765960; E-mail: liulili1203@126.com; ORCID: 0000-0001-8613-8171

