Thermodynamics, kinetics and mechanism analysis of Cu(II) adsorption by in-situ synthesized struvite crystal
来源期刊:中南大学学报(英文版)2018年第5期
论文作者:唐崇俭 彭聪 CHAI Li-yuan(柴立元) 宋雨夏 闵小波
文章页码:1033 - 1042
Key words:struvite; heavy metal; chemical adsorption; coordination bonding; crystal synthesis
Abstract: Synthesized struvite was innovatively applied to removing Cu(II) from aqueous solution. The Cu(II) adsorption behavior and relative mechanisms were studied and analyzed. The maximum Cu(II) adsorption under pH=4.0 and 318 K calculated from adsorption thermodynamic analysis was 145.1 mg/g. The sorption kinetics can be favorably described by pseudo-second order model. The activation energy (Ea) of 17.5 kJ/mol suggested that the adsorption process was a chemical adsorption. The calculated thermodynamic parameters indicated that the adsorption was a spontaneous and endothermic one. On the basis of characterization upon struvite before and after adsorption, it was found that the electrostatic attraction and coordination bonding supported the ion sorption on struvite surface, and the transformation of copper ion into copper hydroxide occurred on struvite surface and within its crevices.
Cite this article as: PENG Cong, CHAI Li-yuan, SONG Yu-xia, MIN Xiao-bo, TANG Chong-jian. Thermodynamics, kinetics and mechanism analysis of Cu(II) adsorption by in-situ synthesized struvite crystal [J]. Journal of Central South University, 2018, 25(5): 1033–1042. DOI: https://doi.org/10.1007/s11771-018-3803-y.

J. Cent. South Univ. (2018) 25: 1033-1042
DOI: https://doi.org/10.1007/s11771-018-3803-y
PENG Cong(彭聪)1, 2, CHAI Li-yuan(柴立元)1, 2, SONG Yu-xia(宋雨夏)1, 2,MIN Xiao-bo(闵小波)1, 2, TANG Chong-jian(唐崇俭)1, 2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metal Pollution, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: Synthesized struvite was innovatively applied to removing Cu(II) from aqueous solution. The Cu(II) adsorption behavior and relative mechanisms were studied and analyzed. The maximum Cu(II) adsorption under pH=4.0 and 318 K calculated from adsorption thermodynamic analysis was 145.1 mg/g. The sorption kinetics can be favorably described by pseudo-second order model. The activation energy (Ea) of 17.5 kJ/mol suggested that the adsorption process was a chemical adsorption. The calculated thermodynamic parameters indicated that the adsorption was a spontaneous and endothermic one. On the basis of characterization upon struvite before and after adsorption, it was found that the electrostatic attraction and coordination bonding supported the ion sorption on struvite surface, and the transformation of copper ion into copper hydroxide occurred on struvite surface and within its crevices.
Key words: struvite; heavy metal; chemical adsorption; coordination bonding; crystal synthesis
Cite this article as: PENG Cong, CHAI Li-yuan, SONG Yu-xia, MIN Xiao-bo, TANG Chong-jian. Thermodynamics, kinetics and mechanism analysis of Cu(II) adsorption by in-situ synthesized struvite crystal [J]. Journal of Central South University, 2018, 25(5): 1033–1042. DOI: https://doi.org/10.1007/s11771-018-3803-y.
1 Introduction
The presence of heavy metals in water sources is generally associated with the disposal of industrial wastes [1–3]. The heavy metals could lead to special concern because of their severe toxicity for human and animal lives [4–6]. The heavy metals were arisen from smelting, alloy manufacturing, electroplating, plastic, mining and refining industries [7, 8]. The traditional methods for heavy metal removal from wastewater include chemical precipitation, ion exchange, membrane method, and adsorption methods using active carbon, ferric oxides, and so forth [9, 10]. These methods are usually inefficient or costly, particularly for removing heavy metals from low- concentration wastewater [11, 12].
It is important to adopt feasible, efficient, and economical method for heavy metal removal. Adsorption method using low-cost solid materials as an adsorbent for heavy metal removal from aqueous state is emerging as a promising alternative to some traditional methods [13–15].For example, some low-cost solid materials have been studied, such as fly ash, lignite, red loess, kaolinite-based clays, red muds, sawdust, loess soil, husk of wheat and rice, and so forth [16, 17]. These adsorption materials can be easily acquired from nature, from industrial processes, or from agricultural activities. Before being applied for wastewater treatment, these materials were always treated or modified. The usual procedure was to graft adsorption groups such as hydroxyl groups, carboxyl groups, ammonia groups, and so forth, to the surface of the materials [18]. The material-modification procedure is always complicated and time-consuming, and the modified material was still difficult to obtain efficient adsorption performance. So, it is urgent to find the adsorption material that is easy to acquire, and has efficient adsorption property.
Herein we report a struvite-based material in-situ synthesized by solution method for the adsorption of heavy metal from wastewater. Struvite is a phosphate mineral with formula of NH4MgPO4·6H2O. It is a mineral commonly found as a product of organic decay in putrescent matter, canned foods or other bacterial action on organic compounds [19]. In our previous researches, the struvite formed in the solution was able to remove ammonia from solution, which was superior than biological method considering the heavy metal inhibition to the microbial activity [20–22]. But surprisingly it was found that the heavy metals can be simultaneously removed along with ammonia during struvite formation process [23]. It was hypothesized that the functional groups including hydroxyl, amino, phosphate present in struvite minerals have certain affinity for heavy metal cations to form heavy metal complexes.
However, the specific adsorption of struvite towards heavy metals such as copper still remains unclear. So, the objective of this work is to evaluate the adsorption properties of struvite for Cu(II) in aqueous solution. A facile solution-phase crystallization control approach was made for synthesis of struvite micron-particles with controllable diameters. The synthesized struvite was used to study adsorption of Cu(II) under controlled conditions. The thermodynamics, kinetics and mechanism analysis toward the Cu(II) adsorption by struvite crystals were conducted.
2 Experimental
2.1 Reagents
All reagents used in this study were of analytical grade and were used without further purification. Ultrapure water (18M) was used in all experiments. Concentrated sulfuric acid (H2SO4), ammonium sulfate ((NH4)2SO4), magnesium chloride hexahydrate (MgCl2·6H2O), disodium hydrogen phosphate (Na2HPO4), and sodium hydroxide (NaOH) were purchased from the Sinopharm Group Chemical Reagent Co., Ltd. Cu(II) was used in the form of CuSO4. The concentration of Cu(II) in this study was 100 mg/L, which was quite common in real polluted water.
2.2 Synthesis of struvite and crystallization control
The synthesis method is based on the crystallization of struvite by adding phosphate salts and magnesium salts into ammonia solution. The struvite crystallization control is targeted at different physical properties including particle size and specific area, in order to screen the optimal synthesis condition of struvite for Cu(II) sorption.
2 g/L ammonia chloride solution (count by N, 1 L) was prepared in 3 L-beaker. Afterwards,1 mol/L Na2HPO4 and MgCl2 solutions were added into the ammonia solution. The addition amount of Na2HPO4 and MgCl2 was based on molar ratio of n(N):n(Mg):n(P)=2:1:1. The effects of stirring time, stirring speed, and NaOH addition speed to the particle sizes of synthesized struvite were investigated. Firstly of all, the reaction time for the synthesis of struvite was controlled as 10 min. The stirring time 2, 4, 6, 8 and 10 min was selected and stirring speed 100, 200, 300, 400 and 500 r/min was adopted. The total amount of NaOH added into the system was 58.1 mL, to realize the final pH value in the system of around 9.0. The pH adjustment rate control was realized by controlling addition speed of NaOH into the system. Firstly, the one-shot addition of NaOH (time for addition was assumed as zero) was applied, and slow addition with rate of 5.8, 7.3, 9.7, 14.5 and 29.1 mL/min was also selected, corresponding to NaOH addition time of 2, 4, 6, 8 and 10 min. The effect of the three factors on struvite physical property was investigated. After reaction, the struvite was rinsed with ultrapure water 3–5 times, and then it was dried at 60 °C for 2 h. The final product under optimal condition in this study was used for Cu(II) adsorption research.
2.3 Adsorption experiment procedure
2.3.1 Effect of pH on Cu(II) adsorption
The effect of pH on the adsorption of Cu(II) by struvite was studied over pH range from 2.0 to 8.0. The pH value of the Cu(II) solution was adjusted by adding H2SO4 (0.01 mol/L) as required. After pH adjustment, 1 g synthesized struvite (10 min stirring, 500 r/min, and fast NaOH addition) was added into the 1 L Cu(II) solution (100 mg/L) at different pH values. The system was stirred at 300 r/min and 298 K for 10 min in order to realize adsorption equilibrium. Then the liquid was filtered by a 0.45 mm membrane filter. The removal rate of Cu(II) (R) and amount of Cu(II) adsorbed by struvite (Q) was determined by the following equations:
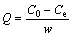 (1)
(1)
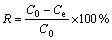 (2)
(2)
where C0 is the initial concentration of Cu(II) (mg/L); Ce is the equilibrium concentration (mg/L); w is the adsorbent amount (g) added into the solution.
2.3.2 Kinetic and thermodynamics experiments
Adsorption kinetic and thermodynamic experiments were conducted to determine the adsorption kinetic and thermodynamic properties of synthesized struvite on Cu(II).Twenty conical flasks (200 mL) with 100 mL Cu(II) solution (100 mg/L, pH=4.0) were prepared. Then the flask was placed in a water bath with temperature of 278 K and 318 K, and was stirred with magnetic stirring at 200 r/min. 0.1 g struvite was added into the flask under stirring condition, and the flask was taken after 5 to 100 s from sample 1 to sample 20, respectively, at time interval of 5 s. Then the sample was filtered through a 0.45 mm membrane filter and the concentration of aqueous Cu(II) was measured.
2.4 Analyzing and characterization methods
The concentration of the Cu(II) in aqueous solution was measured by inductively coupled plasma (ICP-AES, IRIS Intrepid II XSP ThermoFisher). The pH value was measured by Mettler FE20K pH meter.
Zeta potential of synthesized struvite was measured under pH range of 3.0 to 8.0, on the Malvern Zetasizer Nano ZS90 equipment using graphite electrodes. FTIR spectra of struvite before and after Cu(II) adsorption were recorded from 400 cm–1 to 4000 cm–1, via Nicolet IS10 infrared spectrometer with resolution of 4 cm–1. X-ray photoelectron spectroscopy (XPS) analysis upon struvite and Cu(II)-loaded struvite was also conducted on a Thermo Fisher Scientific K-Alpha 1063 using Al-Ka X-ray as the excitation source with analysis chamber ≤10–10 Torr. SEM photos before and after Cu(II) adsorption were also collected by FEI Quanta 650 FEG scanning electron microscopy (SEM), with accelerating voltages of 20 kV. The particle size distribution (PSD) of all samples was determined using Mastersizer 2000 laser particle analyzer (Malvern Instruments Ltd., UK).
3 Results and discussion
3.1 Effect of stirring speed, stirring time, and NaOH addition time on particle size of struvite
The surface area has great impact on the adsorption property of adsorbent, and higher surface area could usually enhance the adsorption performance. So, increasing surface area was an important consideration in application of adsorbent. Since the particle size is inversely correlated with surface area, namely lower particle size means higher surface area. The experimental conditions including stirring time, stirring speed, and NaOH addition speed might have effect on the crystallization process of struvite. So, their influence upon the particle sizes of struvite was investigated. The results are shown in Figure 1. The parameters D(10), D(25), D(75), D(90) point to a specific particle size values: the percentages of the particles with sizes lower than this specific value are 10%, 25%, 75%, and 90% respectively. According to Figure 1(a), the particle size slightly decreased with the increase of stirring time, and stirring time 10 min had the minimum particle size, where the mean surface area was 4.17 m2/g.Figure 1(b) indicates that increasing the stirring speed had no significant effect for the decrease of small particle sizes (smaller than D(10) and D(25)), but had visible effect for higher particle sizes, as shown in D(75) and D(90). Compared with effect of stirring speed and stirring time, NaOH addition speed had great impact on the particle size. As shown in Figure 1(c), the decrease of NaOH addition speed led to increase in particle size, suggesting that reducing the addition time could lower the particle size. The one-shot addition, namely addition time was assumed as zero, had the minimum particle size, with mean surface area of 23.42 m2/g. So, it is clear that the optimum synthesis conditions were stirring time of 10 min, stirring speed of 500 r/min, and NaOH addition in one-shot manner. This optimal condition was selected for synthesis of struvite applied for Cu(II) adsorption. The synthesized struvite was shown in scanning electronic microscopy (SEM) photo (Figure 2(a)). It can be seen that the struvite synthesized in this experiment was prism-shaped, and was abundant with visible crevices. The crevices could be favorable for the adsorption of metal ions since it can enhance the surface area.
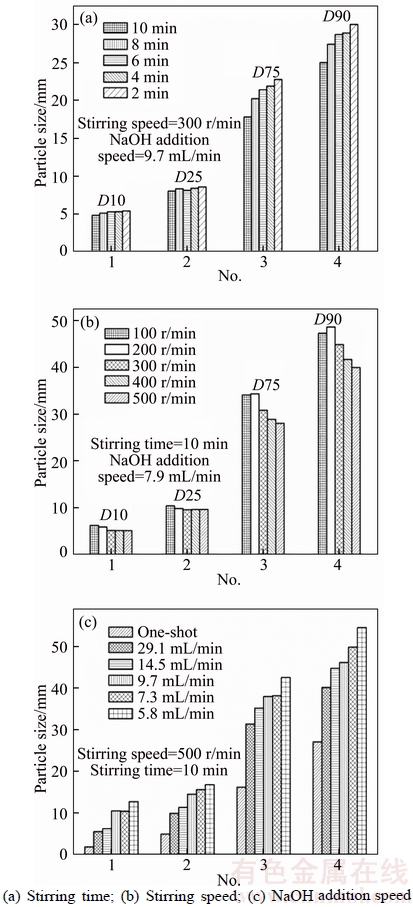
Figure 1 Effect of different factors on particle size of struvite
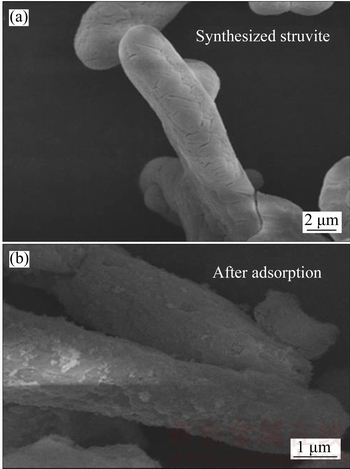
Figure 2 SEM photos of synthesized struvite (a) and struvite after adsorption of Cu(II) (b)
3.2 Effect of pH on adsorption of Cu(II)
It is integral to consider the effect of pH on the results of Cu(II) adsorption by struvite since pH value can influence the metal species in the solution and the functional groups on the surface of adsorbent [24]. The effect of pH on Cu(II) adsorption results is shown in Figure 1(a) over pH range of 2.0 to 8.0.
As seen in Figure 3(a), the Cu(II) adsorption on struvite increased with increasing the initial pH value of the solution, and it reached to peak at pH 4.0 and 5.0. The maximum removal rate was realized at pH 4.0, with removal of 99.9%. Afterwards, it slightly went down within pH of 5.0 to 8.0, and then it decreased to 92.9% at pH 8.0. It suggests that increasing pH value after 5.0 would lead to decrease in Cu(II) removal efficiency. This result can be suitably explained by the change of copper species in the solution. As shown in Figure 3(b), which was drawn under support of Visual MINTEQ, the proportion of aqueous Cu2+ decreased with the increase of pH. The copper existed predominantly as Cu2+ in solution within pH 2.0 to 5.0. At pH 7.0, the proportion of Cu(OH)2 and CuOH+ increased to 64.4% and 27.6%, respectively. So, it is easy to conclude that the formation of copper hydroxide and copper complexes prior to the struvite’s adsorption would impede the adsorption efficiency. The optimal initial solution pH value scope for Cu(II) adsorption on struvite ranged from 4.0 to 5.0.
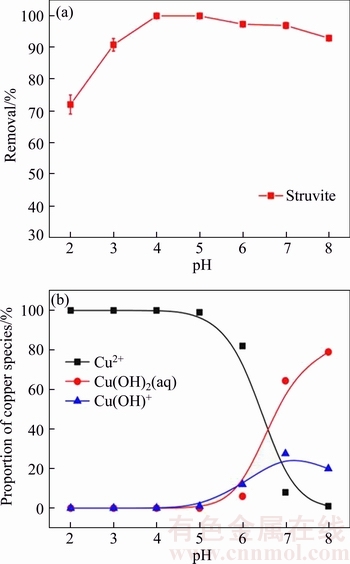
Figure 3 Effect of pH on adsorption of Cu(II) (a) and copper species (b) in solution over pH range of 2.0 to 8.0
3.3 Adsorption kinetics and thermodynamics
3.3.1 Adsorption kinetics
The sorption kinetics of Cu(II) on struvite was studied through a span of contact time at temperatures of 278 K and 318 K, respectively. Based on analysis via different kinetic models, it was found that the experimental data on kinetics of Cu(II) adsorption on struvite can be well fitted to pseudo-second-order kinetic models [25–27]:
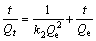 (3)
(3)
where Qe and Qt are the adsorption amount of Cu(II) (mg/g) at equilibrium and at time t(min), respectively; k2 is the rate constant of pseudo- second-order kinetic equation.
Figure 4 shows the linear regression analysis upon fitting t and t/Qt. The fitting kinetic parameters calculated based on linear fitting are listed in Table 1. It is clearly seen that the pseudo-second-order model with the determined coefficient (R2) of higher than 0.99 can well describe the adsorption of Cu(II) on struvite at 298–318 K.

Figure 4 Pseudo-second-order kinetic fitting against plots of Cu(II) adsorption by struvite
The activation energy (Ea) of Cu(II) adsorption on struvite could be calculated using the Arrhenius equation as follows [28, 29]:
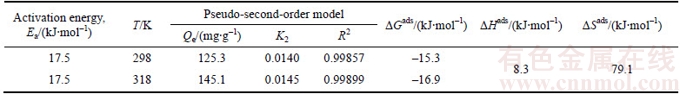
Table 1 Kinetic and thermodynamic parameters for adsorption of Cu(II) on struvite at 298 K and 318 K
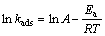 (4)
(4)
where Ea is the activation energy (kJ/mol); kads is the adsorption rate constant; A is the Arrhenius constant; R is the gas constant with value of 8.314 J/(mol·K); T is the thermodynamic temperature (K).
Ea can indicate the type of adsorption: the activation energy for physical adsorption is normally lower than 4.2 kJ/mol, while the activated chemical adsorption is usually in a range of 8.4–83.7 kJ/mol [28]. On the basis of kinetic analysis above, Ea value was determined from the slope of Arrhenius plot as listed in Table 1. Ea value of 17.5 kJ/mol (Table 1) indicates that Cu(II) adsorption by struvite is a chemical process.
3.3.2 Adsorption thermodynamics
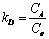 The temperature influence on the adsorption process is related to the thermodynamic parameters including Gibb’s free energy change (△Gads), entropy change (△Sads), and enthalpy change (△Hads). The parameters for struvite’s sorption of Cu(II) (with concentration of 100 mg/L) can be estimated by the equations as follows [30]:
The temperature influence on the adsorption process is related to the thermodynamic parameters including Gibb’s free energy change (△Gads), entropy change (△Sads), and enthalpy change (△Hads). The parameters for struvite’s sorption of Cu(II) (with concentration of 100 mg/L) can be estimated by the equations as follows [30]:
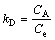 (5)
(5)
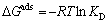 (6)
(6)
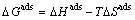 (7)
(7)
It can be deduced from Eqs. (5)–(7):
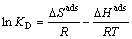 (8)
(8)
where KD is distribution coefficient and can be determined by Eq. (5); CA and Ce are the equilibrium amount of Cu(II) on adsorbent and in the solution respectively, with unit of mg/L; and R is the gas constant with value of 8.314 J/(mol·K). △Hads and △Sads can be calculated from slope and intercept of linear equation of lnKD vs 1/T, and are listed in Table 1. As shown in Table 1, the Gibbs free energies for Cu(II) adsorption process under both 298 K and 318 K are negative, which indicates that the Cu(II) adsorption process was thermodynamically realizable and can be spontaneous at the temperatures in the experiments. The enthalpy change (△Hads) for Cu(II) adsorption is 8.3 kJ/mol, suggesting that the sorption of Cu(II) by struvite is an endothermic process. Moreover, the entropy change (ΔSads) is 79.1 J/(mol·K), indicating that the randomness at the solid-solution increased during the adsorption process [30].
3.4 Possible mechanism for Cu(II) adsorption by struvite
Kinetic studies and thermodynamic studies have provided some information related to the chemical and endothermic adsorption of Cu(II) onto synthesized struvite. The specific mechanism for the enhanced Cu(II) removal by struvite was not quite clear. Thus, further studies corresponding to the Cu(II) adsorption have been conducted.
The SEM comparison photos (Figure 2) show that after Cu(II) adsorption the struvite was filled with small particles on its surface and in the crevices. XPS was used to determine the existing form of elements before and after adsorption. The XPS spectra are illustrated in Figures 5(a) and (b). As shown in Figure 5(a), after adsorption, some peaks were clearly identified at 920–960 eV, which was arisen from Cu(II) element adsorption. Through peak separation upon the Cu 2p (Figure 5(b)) spectrum, it is clear that the peaks were assigned to Cu(OH)2 (935.1 eV and 937.4 eV for Cu 2p3/2 and 939.1, 942.0, 944.2 eV for satellite peak) and another Cu(II) existing form [31], most probably copper complex in this experiment. The corresponding proportions of Cu(OH)2 and copper complex were 94.5% and 5.5%, respectively, via peak fitting calculation. So, it can be inferred that after Cu(II) adsorption, it was filled with Cu(OH)2 on its surface and in the crevices. Through FTIR comparison (Figure 5(c)), it is clear that before adsorption there were two weak peaks at ~1630 and ~1685 cm–1, assigning to the bending vibration of O—H in adsorption water and O—H in crystal water, respectively [32]. The strong double peak at~1436 cm–1 was related to the N—H vibration in ammonia group. After adsorption, the peak attributed to O—H in adsorption water shifted to lower wavenumber at ~1603 cm–1. Moreover, both the adsorption peak of O—H in adsorption water and the peak attributed to N—H were widened. This information provided proofs that hydrogen bonding existed between Cu(OH)2 and struvite [33]. Furthermore, struvite zeta potentials under different pH (Figure 5(d)) provided information that struvite was negatively charged under pH range of higher than 3.0, indicating that the electrostatic attraction between struvite and Cu(II) ions can occur during the adsorption process. It can be deduced from the above results that the Cu(II) adsorption process has three main stages (Figure 6).
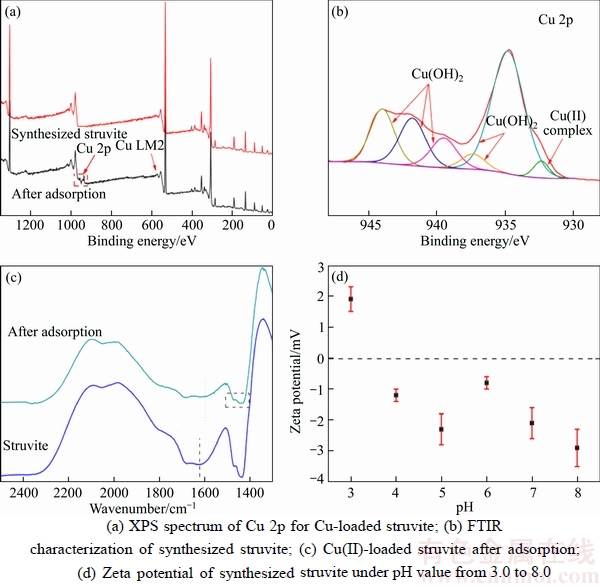
Figure 5 XPS spectra of struvite and Cu-loaded struvite
In the first stage (Stage 1), the copper ions with unoccupied orbit were coordinated with —NH3, —OH, and PO43– on the surface of struvite. Moreover, the ions were attracted to the surface of struvite under electrostatic force. This could be called Cu(II) accumulation process on the surface of struvite. In the second stage (Stage 2), the increasingly high concentration of Cu(II) on struvite’s surface provided chance of Cu(OH)2 formation after bonding with aqueous OH–, under pH higher than 4.0. The newly formed Cu(OH)2 in molecule-scale had hydrogen bonding with amino groups and hydroxyl groups on struvite surface. In the third stage (Stage 3), as Cu(OH)2 was increasingly formed on struvite’s surface, the nucleation of copper hydroxide occurred and the nucleus continuously grew until particles can be clearly observed.
4 Conclusions
1) The maximum Cu(II) adsorption on struvite under pH=4.0 and 318 K calculated from adsorption thermodynamic analysis is 145.1 mg/g.
2) The sorption kinetics fitted well to pseudo- second order model. The calculated activation energy (Ea) of 17.5 kJ/mol indicates the chemical adsorption. The Gibbs free energy (–15.3 to –16.9 kJ/mol) and enthalpy change (8.3 kJ/mol) indicate that the adsorption is spontaneous and endothermic process.
3) The electrostatic attraction and coordination bonding support the ion sorption on struvite surface, and the hydrogen bonding arisen from struvite’s ammonia and hydroxyl group with Cu(OH)2 molecules occurs. The copper ions are finally transformed into visible copper hydroxide crystals on struvite surface.
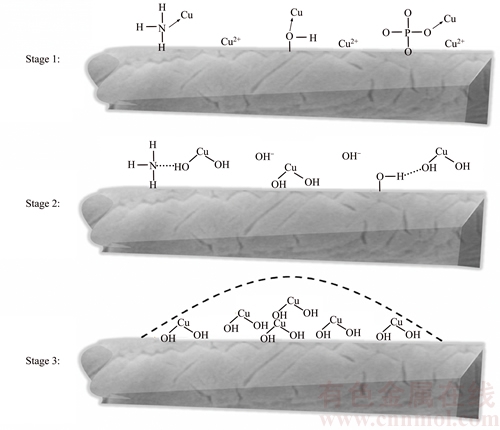
Figure 6 Sketch mechanisms of removal of Cu(II) from solution by synthesized struvite
References
[1] WANG T, ZHANG L, LI C, YANG W, SONG T, TANG C, MENG Y, DAI S, WANG H, CHAI L, LUO J. Synthesis of Core-shell magnetic Fe3O4@poly(m-phenylenediamine) particles for chromium reduction and adsorption [J]. Environmental Science & Technology, 2015, 49(9): 5654–5662.
[2] YANG J, CHAI L Y, WANG Y Y, HE X W, WANG J L. Transportation and distribution of chromium in the anaerobic sludge treating the chromium-containing wastewater [J]. International Journal of Environment & Pollution, 2010, 38(3): 256–266.
[3] JIANG B F, SUN W L. Assessment of heavy metal pollution in sediments from Xiangjiang River (China) using sequential extraction and lead isotope analysis [J]. Journal of Central South University, 2014, 21: 2349–2358.
[4] WANG Y, PENG B, YANG Z, TANG C, CHEN Y. Treatment of Cr(VI) contaminated water with Pannonibacterphragmitetus BB [J]. Environmental Earth Sciences, 2014, 71(10): 4333–4339.
[5] FEI J C, MIN X B, WANG Z X, PANG Z H, LIANG Y J, KE Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case study from South China [J]. Environmental Science and Pollution Research, 2017, 24(35): 27573–27586.
[6] LIU D G, MIN X B, KE Y, CHAI L Y, LIANG Y J, LI Y C, YAO L W, WANG Z B. Co-treatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: solidification/stabilization of arsenic and heavy metals with minimal cement clinker [J]. Environmental Science and Pollution Research, 2018. DOI: 10.1007/s11356-017-1084-x.
[7] HUANG S, YUAN C, LI Q, TANG C, OUYANG K, WANG B. Distribution and risk assessment of heavy metals in soils from a typical Pb-Zn mining area [J]. Polish Journal of Environmental Studies, 2017, 26(3):1105–1112.
[8] KONG X F, YANG B, XIONG H, ZHOU Y, XUE S G, XU B Q, WANG S X. Selective removal of heavy metal ions from aqueous solutions with surface functionalized silica nanoparticles by different functional groups [J]. Journal of Central South University, 2014, 21: 3575–3579.
[9] TANG C, DUAN C, YU C, SONG Y, CHAI L, XIAO R, WEI Z, MIN X. Removal of nitrogen from wastewaters by anaerobic ammonium oxidation (ANAMMOX) using granules in upflow reactors [J]. Environmental Chemistry Letters, 2017, 15(2): 311–328.
[10] ZENG J, YE H, HUANG N, LIU J, ZHENG L. Selective separation of Hg(II) and Cd(II) from aqueous solutions by complexation–ultrafiltration process [J]. Chemosphere, 2009, 76(5): 706–710.
[11] CHEN Y, YE W, YANG X, DENG F, HE Y. Effect of contact time, pH and ionic strength on Cd (II) adsorption from aqueous solution onto bentonite from Gaomiaozi, China [J]. Environmental Earth Sciences, 2011, 64(2): 329–336.
[12] CHEN Y, PENG L, ZENG Q, YANG Y, LEI M, SONG H, CHAI L, GU J. Removal of trace Cd(II) from water with the manganese oxides/ACF composite electrode [J]. Clean Technologies and Environmental Policy, 2015, 17(1): 49–57.
[13] PENG L, CHEN Y, DONG H, ZENG Q, SONG H, CHAI L, GU J. Removal of trace As(V) from water with the titanium dioxide/ACF composite electrode [J]. Water, Air, & Soil Pollution, 2015, 226(7): 1–11.
[14] JING Q, CHAI L, HUANG X, TANG C, GUO H, WANG W. Behavior of ammonium adsorption by clay mineral halloysite [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1627–1635.
[15] WANG Y, LI Z W, HUANG B, JIANG W G, GUO L, HUANG J Q, ZENG G M. Kinetics comparison on simultaneous and sequential competitive adsorption of heavy metals in red soils [J]. Journal of Central South University, 2015, 22: 1269–1275.
[16] XIAO R, GAO L, WEI Z, SPINNEY R, LUO S, WANG D, DIONYSIOU D, TANG C, YANG W. Mechanistic insight into degradation of endocrine disrupting chemical by hydroxyl radical: An experimental and theoretical approach [J]. Environmental Pollution, 2017, 231(2): 1446–1452.
[17] CHEN R, CHAI L, LI Q, SHI Y, WANG Y, MAHMOOD A. Preparation and characterization of magnetic Fe3O4/CNT nanoparticles by RPO method to enhance the efficient removal of Cr(VI) [J]. Environmental Science and Pollution Research, 2013, 20(10): 7175–7185.
[18] HAO S Y, VERLOTTA A, APREA P, PEPE F, CAPUTO D, ZHU W. Optimal synthesis of amino-functionalized mesoporous silicas for the adsorption of heavy metal ions [J]. Microporous and Mesoporous Materials, 2016, 236: 250–259.
[19] LIN J B, YUAN S J, WANG W, HU Z H, YU H Q. Precipitation of organic arsenic compounds and their degradation products during struvite formation [J]. Journal of Hazardous Materials, 2016, 317: 90–96.
[20] PENG C, CHAI L, TANG C, MIN X, SONG Y, DUAN C, YU C. Study on the mechanism of copper-ammonia complex decomposition in struvite formation process and enhanced ammonia and copper removal [J]. Journal of Environmental Sciences, 2017, 51: 222–233.
[21] SONG Y X, CHAI L Y, TANG C J, XIAO R, LI B R, WU D, MIN X B. Influence of ZnO nanoparticles on anammox granules: The inhibition kinetics and mechanism analysis by batch assays [J]. Biochemical Engineering Journal, 2018, 133: 122–129. DOI: 10.1016/j.bej.2018.02.006.
[22] CHAI L, PENG C, MIN X, TANG C, SONG Y, ZHANG Y, ZHANG J. Two-sectional struvite formation process for enhanced treatment of copper-ammonia complex wastewater [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 457-466.
[23] Peng C, Chai L, Tang C, Min X, Ali M, Song Y, QI W. Feasibility and enhancement of copper and ammonia removal from wastewater using struvite formation: A comparative research [J]. Journal of Chemical Technology and Biotechnology, 2017, 92: 325–333.
[24] SHENG P X, TING Y P, CHEN J P, HONG L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms [J]. Journal of Colloid and Interface Science, 2004, 275: 131–141.
[25] Ho Y S, Mckay G. A Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents [J]. Process Safety and Environment Protection, 1998, 76: 332–340.
[26] SONG Y, LIAO Q, YU C, XIAO R, TANG C, CHAI L, DUAN C. Physicochemical and microbial properties of settled and floated anammox granules in upflow reactor [J]. Biochemical Engineering Journal, 2017, 123: 75–85.
[27] CHAI L, WANG Y, ZHAO N, YANG W, YOU X. Sulfate- doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water [J]. Water Research, 2013, 47: 4040–4049.
[28] SMITH J M. Chemical engineering kinetics [M]. New York: McGraw-Hill, 1970.
[29] YE T, WEI Z, SPINNEY R, TANG C, LUO S, XIAO R, DIONYSIOU D. Chemical structure-based predictive model for the oxidation of trace organic contaminates by sulfate radical [J]. Water Research, 2017,116(1): 106–115.
[30] Kadirvelu K, Namasivayam C. Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd(II) from aqueous solution [J]. Advances in Environmental Research, 2004, 7: 471–478.
[31] Chawla S K, Sankarraman N, Payer J H. Diagnostic spectra for XPS analysis of Cu—O—S—H compounds [J]. Journal of Electron Spectroscopy and Related Phenomena, 1992, 61: 1–18.
[32] Gayan R, Grassian V H. Role(s) of adsorbed water in the surface chemistry of environmental interfaces [J]. Chemical Communications, 2013, 49: 3071–3094.
[33] Okur H I, Kherb J, Cremer P S. Cations bind only weakly to amides in aqueous solutions [J]. Journal of American Chemical Society, 2013, 135: 5062–5067.
(Edited by YANG Hua)
中文导读
鸟粪石晶体吸附Cu(II)的热力学和动力学特征分析及机制探讨
摘要:本文采用基于液相原位调控合成的鸟粪石晶体材料开展了对Cu(II)的吸附研究。基于吸附热力学及动力学分析,揭示了鸟粪石晶体对Cu(II)吸附行为及相关机理。合成的鸟粪石晶体材料属斜方晶系,颗粒表面分布有较多孔隙、狭缝,比表面积为23.42 m2/g。热力学分析表明,鸟粪石晶体材料在pH 4.0及318 K条件下对Cu(II)的饱和吸附量为145.1 mg/g;吸附动力学分析表明,吸附过程符合准二级动力学模型。经计算可得其吸附活化能Ea为17.5 kJ/mol,表明吸附过程属于化学吸附。此外,进行了包括吉布斯自由能变ΔGads 、焓变ΔHads、熵变ΔSads在内的热力学计算,结果表明鸟粪石晶体吸附Cu(II)过程属于自发、吸热反应。基于对鸟粪石晶体材料吸附前、后的表征,发现过程中表面静电引力及配位作用促进了铜离子在鸟粪石表面的吸附,并且发现鸟粪石晶体材料在表面及裂缝中存在有铜离子向氢氧化铜的转变行为。
关键词:鸟粪石;重金属;化学吸附;配位作用;晶体合成
Foundation item: Project(51674305) supported by the National Natural Science Foundation of China; Project(2013WK2007) supported by the Key Project of Science and Technology of Hunan Province, China; Project(2015CX001) supported by the Innovation Stimulating Program of Central South University, China
Received date: 2016-10-12; Accepted date: 2017-02-24
Corresponding author: TANG Chong-jian, PhD, Associate Professor; Tel: +86–731–88830511; Fax: +86–731–88710171; E-mail: chjtang@csu.edu.cn

