J. Cent. South Univ. (2012) 19: 863-868
DOI: 10.1007/s11771-012-1084-4
Large scale synthesis of ZnO nanoparticles via homogeneous precipitation
WANG Yi-ming(王益明)1, LI Jian-hua(李建华)2, HONG Ruo-yu(洪若瑜)1, 3, 4
1. Chemical Engineering and Materials Science & Key Laboratory of Organic Synthesis of Jiangsu Province,
College of Chemistry, Soochow University, Suzhou 215123, China;
2. Kailuan Zhongrun Coal Chemical Co., Ltd., Tangshan 063611, China;
3. State Key Laboratory of Multi-phase Complex Systems, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100080, China;
4. College of Chemistry and Chemical Engineering, Fuzhou University, Fuzhou 350108, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: In order to synthesize ZnO nanoparticles economically, industrial-grade zinc sulfate and urea were utilized to synthesize ZnO precursors in a stirred-tank reactor or a Teflon-lined autoclave at 100-180 °C under complete sealing condition. The ZnO precursors were calcined at 450 °C for 3 h to synthesize ZnO nanoparticles. The composition of the precursors and the formation mechanism of ZnO were studied by thermogravimetric analysis and Fourier transform infrared spectroscopy. The results of X-ray diffraction, transmission electron microscopy and scanning electron microscopy of the ZnO powders demonstrate that high-purity zincite ZnO nanoparticles are synthesized. Orthogonal experiments were performed to find out the optimal conditions for the maximum yield and the minimum size. The effect of temperature on the size of ZnO nanoparticles was investigated. The results show that a higher temperature is propitious to obtain smaller nanoparticles.
Key words: ZnO; homogeneous synthesis; sealing condition; thermogravimetric analysis
1 Introduction
Zinc oxide (ZnO) with excellent electronic, optical and photocatalytic properties [1-3] has attracted much attentions in recent decades. Many methods, such as homogeneous precipitation [4], sol-gel processing [5], hydrothermal synthesis [6-7], vapor deposition [8], decomposition of organometallic precursors [9], gas expanding method [10], vapor transportation [11], hydrothermal oxidative pressure-relief route [12], solvothermal hot press (STHP) method [13] and interphase synthesis [14], have been developed to synthesize ZnO nanoparticles. Several morphologies of ZnO nanoparticles, e.g. nanorings [15], nanohelices [16], nanowire or nanobelt [17], flowerlike nanorod [18-19], nanotubes [20] and cone-shaped nanoparticles [21], have been obtained.
For the deposition method, oxygen pressure is a significant parameter on the formation and morphology of ZnO nanoparticles. Using pulsed laser deposition, QI et al [22] studied the effects of oxygen pressure on crystallinity and surface morphology of ZnO films, and found that the surface morphologies depend significantly on the oxygen partial pressure. SINGH et al [23] reported the influence of oxygen partial pressure on the structural properties of the as-grown ZnO nanocrystalline thin films, and found that the films deposited at low oxygen partial pressure contained mixed phase (Zn and ZnO) and were randomly oriented while the films deposited at higher oxygen partial pressure were single phase (ZnO) and highly oriented along the c-axis. In the homogeneous precipitation, precipitators decomposed into acidic and/or alkali gases [24-25] at a high temperature, and the gases streamed out inevitably in the three-neck flask, and the yield of products became low. Therefore, it is necessary to reduce the loss of the gases in order to obtain a higher yield.
In this work, ZnO nanoparticles were synthesized utilizing industrial-grade raw materials via homogeneous precipitation under complete sealing condition. The composition of ZnO precursors and the formation mechanism of ZnO nanoparticles were studied via thermogravimetric analysis and Fourier transform infrared spectroscopy. The effect of temperature on the size of ZnO nanoparticles was investigated.
2 Experimental
2.1 Reagents and purification
Industrial grade zinc sulfate (ZnSO4) and urea were utilized and purified before use. Analytical purity zinc powders and potassium permanganate were purchased from Sinopharm Chemical Reagent Co. Ltd.
To obtain high-purity ZnO nanoparticles, the raw materials were purified according to the following procedures: 1) Purification of ZnSO4: Some zinc powders were plunged into zinc sulfate solution with stirring at 40 °C for some time, and the precipitates were filtrated out. Then, the pellucid filtrate was heated to a higher temperature, and some potassium permanganate was mixed into the filtrate subsequentially. One day later, another zinc powders were added, and then the mixture was filtrated. The resulted pellucid solution was used as zinc resource; 2) Purification of urea: Urea was dissolved in deionized water to form a saturated solution. One day later, the precipitates were filtrated out and the obtained transparent filtrate was used as precipitator.
2.2 Synthesis procedure
2.2.1 Synthesis in stirred-tank reactor
The pretreated solutions were injected into a stirred- tank reactor with a volume of 4.5 L. The reaction was performed under complete sealing condition. When the temperature reached 70 °C, the exhaust air was eliminated through a needle valve. Turn on the agitator and adjust the temperature at (100±1) °C. Several hours later, the reaction system was cooled to room temperature. The precipitates were filtrated and washed with ammonia (pH=9.0) and anhydrous alcohol for several times. The filter cake was dried at 100 °C for 2 h and milled. The obtained ZnO precursors were calcined at 450 °C for 3 h to produce ZnO nanoparticles.
2.2.2 Synthesis in Teflon-lined autoclave
The procedure was the same as that described above. The differences were the reaction temperature and the reactor. The reaction temperature varied from 120 to 180 °C and the reaction is performed in a 100 mL Teflon-lined autoclave without stirring.
2.2.3 Orthogonal experiments
Orthogonal experiments were carried out to investigate the influences of reaction time and material ratio on the size and yield of ZnO nanoparticles. The orthogonal experiments were carried out at 100 °C in the stirred-tank reactor. The factors and levels of the orthogonal experiments are listed in Table 1.
2.3 Characterization
The Perkin-Elmer TGA-7 with variable temperatures from 0 to 700 °C and Nicolet Avatar 360 Fourier transform infrared spectroscope (FT-IR) were employed to investigate the thermal behavior and the composition of the ZnO precursors. Crystalline structure of ZnO nanoparticles was characterized by the X-ray diffractometer (XRD) (D/Max-IIIC, Japan) using Cu Kα radiation. Transmission electron microscope (TEM) (Hitachi H-600-II, Japan) and scanning electron microscope (SEM) (Hitachi S-570, Japan) were used to determine the size and shape of ZnO nanoparticles. The size distribution of ZnO nanoparticles/aggregates in alcohol was obtained using Malvern HPPS5001 laser particle-size analyzer (DTS) with the scanning range from 0.6 to 6 000 nm. The purity of ZnO nanoparticles was determined by an accessory (EDAX) of SEM.
Table 1 Factors and levels of orthogonal experiments

3 Results and discussion
3.1 Composition of precursors
Figure 1 shows the TGA spectra of ZnO precursors, which exhibit two obvious mass losses. The first mass loss occurs at about 100 °C and can be attributed to the evaporation of water adsorbed. The second mass loss takes place at 287 °C, and about 23.89% of the total mass is lost.
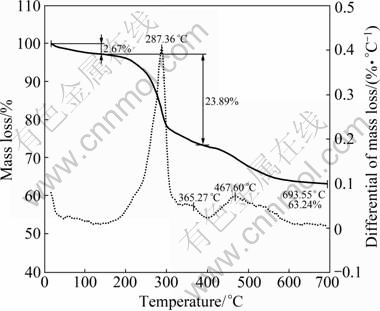
Fig. 1 TGA spectra of ZnO precursors synthesized in stirred- tenk reactor
Figure 2 illustrates the IR spectrum of ZnO precursors. The flexing vibration absorption of hydroxyl (—OH) is assigned to the peak at 3 371 cm-1. The peaks at 1 552 cm-1 and 1 507 cm-1 can be attributed to the flexing vibration of carboxyl (C=O) of carbonate. The peaks at 1 388 cm-1 and 474 cm-1 are due to the flexing vibration of C—O and Zn—O, respectively.
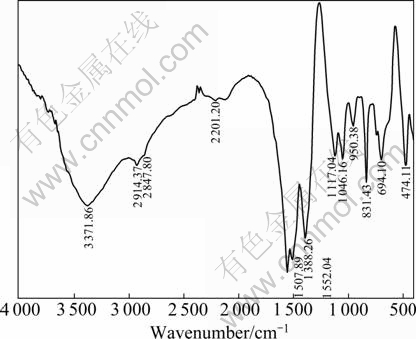
Fig. 2 IR spectrum of ZnO precursors synthesized in stirred reactor
Based on the above description, the ZnO precursors can be imagined to be zinc carbonate hydroxide and the mechanism of producing ZnO nanoparticles can be deduced as follows:
Decomposition of urea:
CO(NH2)2+3H2O=CO2+2NH3·H2O (1)
Precipitation of Zn2+:
CO2+H2O H2CO3
H2CO3 H++HCO-3
H++HCO-3 2H++CO2-3 (2)
2H++CO2-3 (2)
NH3·H2O NH+4+OH- (3)
NH+4+OH- (3)
3Zn2++CO2-3+4OH-+xH2O=[ZnCO3·2Zn(OH)2 ]·xH2O (4)
In Eq. (4), water molecules are assumed to be adsorbed onto the surface of zinc carbonate hydroxide to form [ZnCO3·2Zn(OH)2 ]·xH2O. The adsorbed water is about 2.65% of the total mass. In the furnace of TGA, the following two reactions are performed to produce ZnO nanoparticles:
[ZnCO3·2Zn(OH)2]·xH2O=ZnCO3·2Zn(OH)2+xH2O↑ (5)
ZnCO3·2Zn(OH)2=3ZnO+CO2↑+2H2O↑ (6)
In Eq. (6), the theoretical mass loss is 23.46%, which is almost consistent with the experimental mass loss of 23.89%.
3.2 XRD analysis
Figure 3 illustrates the XRD patterns of the synthesized ZnO nanoparticles. From these patterns, it is found that all the peaks are well indexed to hexagonal crystal according to the standard spectrum of ZnO bulk crystal (Zincite, JCPDS 36-1451), where 2θ values correspond to 31.98° (100), 34.52° (002), 36.30° (101), 47.63° (102), 56.73° (110), 63.00° (103), 66.56° (200), 68.22° (112) and 69.09° (201), respectively. The average crystallite size D is calculated to be about 19 nm, using the Debye- Sherrer formula D=Kλ/(βcosθ), where K is the Sherrer constant, 0.89; λ is the X-ray wavelength, 1.540 6 ?; β is the peak width of half-maximum; θ is the Bragg diffraction angle. There are no other peaks indicating that high-purity ZnO nanoparticles are obtained through this method. Besides, the inserted EDAX spectrum in Fig. 3 also exhibits only zinc and oxygen elements in ZnO powders, which further proves high purity of the synthesized ZnO nanoparticles.
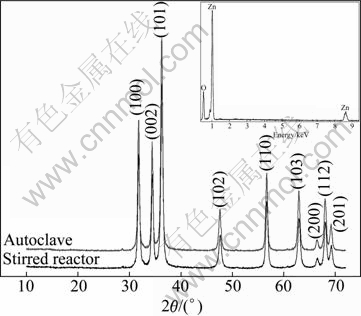
Fig. 3 XRD patterns of ZnO nanoparticles (Inserted EDAX spectrum reveals elements of ZnO nanoparticles synthesized in stirred reactor)
3.3 TEM and DTS analysis
ZnO nanoparticles were dispersed in anhydrous alcohol to prepare dilute suspension, which could be used in the TEM and laser scattering measurements. The transmission electron microscope was used to determine the shape and size of ZnO nanoparticles and laser particle-size analyzer was employed to study the size distribution of ZnO nanoparticles/aggregates in anhydrous alcohol. Figure 4 shows the TEM image of ZnO nanoparticles and reveals that there are some aggregates. Besides, it can also be observed that the shape of ZnO nanoparticles is quasi-sphere and the average diameter of monodispersed ZnO nanoparticles is 18 nm. The average diameter of other ZnO nanoparticles synthesized under different conditions can be calculated, as listed in Table 2.
In the alcohol solution, ZnO nanoparticles tend to congregate due to their large surface area. Figure 5 reveals the size distribution of all ZnO samples in alcohol and the mean size of ZnO aggregates is also listed in Table 2. From Table 2, it can be found that the average size of ZnO nanoparticles varies from 11 nm to 18 nm and the size of ZnO aggregates is between 65 nm and 160 nm. When the ratio of urea to zinc sulfate is 2.5:1 or 3:1 and the reaction time is 2.5 h or 3 h, the minimum particle size of 11 nm can be obtained.

Fig. 4 TEM image of ZnO nanoparticles synthesized in stirred reactor
Table 2 Results of orthogonal experiments (synthesized in stirred reactor)

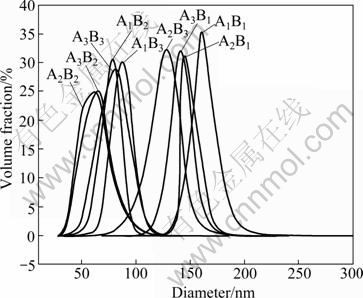
Fig. 5 Size distribution of ZnO nanoparticles
3.4 SEM analysis
The scanning electron microscope was used to observe the morphology of ZnO nanoparticles synthesized in the autoclave at a temperature above the boiling point of water. Figure 6 shows the results of SEM, indicating that the rectangular and net-like ZnO nanoparticles are synthesized. In Fig. 6(a), the length of the rectangle varies from 20 nm to 100 nm. In Fig. 6(b), ZnO nanoparticels link together to form a ZnO nanonet and the average diameter of the meshes is 10 nm. The results of TEM and SEM show that the morphologies of ZnO nanoparticles synthesized under different temperatures differ very much from sphere to rectangle and to nanonet structure.
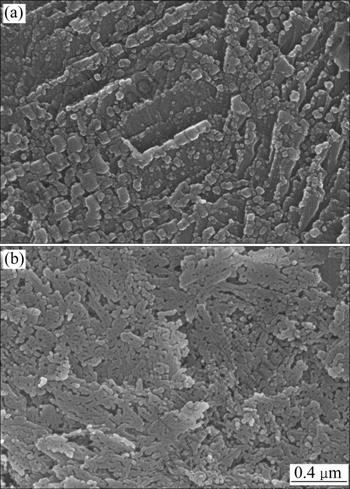
Fig. 6 SEM images of ZnO nanoparticles synthesized in autoclave at 120 °C (a) and 160 °C (b)
3.5 Optimal conditions
The results of orthogonal experiment are listed in Table 2. From Table 2, it can be found that the yield of ZnO nanoparticles increases with the increase of the ratio of urea to zinc sulfate and the reaction time, and the maximal yield is 66.8% when the ratio of urea to zinc sulfate is 3:1 and the reaction time is 3 h. At a lower temperature, urea decomposes incompletely and becomes the obstacle for obtaining a higher yield.
Furthermore, a Teflon-lined autoclave was used to investigate the influence of reaction temperature on the size and yield of ZnO nanoparticles. The results shown in Fig. 7 reveal that the size of ZnO nanoparticles decreases with the increase of temperature, but the yield increases. This can be explained as follows: along with the elevation of temperature, urea decomposes continuously and the supersaturation of ammonia increases rapidly in the reaction system. The particle size is related to the supersaturation of ammonia. The concentration of hydroxyl (OH-) increases with the increase of supersaturation of ammonia that avails both formation and growth of ZnO crystal seeds. When the supersaturation of ammonia comes to an extent, the rate of crystal growth is inferior to that of crystal formation. Thus, smaller particles size can be obtained at a higher supersaturation of ammonia. Due to the continuously concentrated hydroxyl, the yield of ZnO nanoparticles increases with the increase of temperature. Moreover, it can be found that the ZnO nanoparticles synthesized in the autoclave are larger than those synthesized in the stirred reactor, indicating that stirring is propitious to prepare smaller nanoparticles.
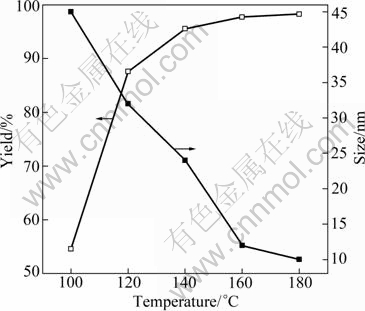
Fig. 7 Relationship of yield and size of ZnO nanoparticles synthesized in autoclave with temperature
4 Conclusions
1) Industrial-grade zinc sulfate purified using zinc powders and potassium permanganate can be utilized to synthesize high-purity ZnO nanoparticles, and a stirrer is propitious to prepare smaller nanoparticles.
2) The yield of ZnO nanoparticles increases with the increase of reaction time, temperature and the ratio of urea to zinc sulfate.
3) These quasi-spherical ZnO nanoparticles are hexagonal crystal. The particle size is influenced by the supersaturation of ammonia, which increases with the reaction temperature. The higher the supersaturation is, the smaller the particle size is. The minimum particle size of about 11 nm can be obtained when the ratio of urea to zinc sulfate is 2.5: 1 or 3:1 and the reaction time is 2.5 h or 3 h.
References
[1] AMRANI B, CHIBOUB I, HIADSI S, BENMESSABIH T, HAMDADOU N. Structural and electronic properties of ZnO under high pressures [J]. Solid State Communications, 2006, 137(7): 395-399.
[2] SANS J A, SEGURA A, MANJ?N F J, MAR? B, MU?OZ A, HERRERA-CABRERA M J. Optical properties of wurtzite and rock-salt ZnO under pressure [J]. Microelectronics Journal, 2005, 36(10): 928-932.
[3] DANESHVAR N, SALARI D, KHATAEE A R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2 [J]. Journal of Photochemistry and Photobiology A, 2004, 162(2/3): 317-322.
[4] KIM J H, CHOI W C, KIM H Y, KANG Y, PARK Y K. Preparation of mono-dispersed mixed metal oxide micro hollow spheres by homogeneous precipitation in a micro precipitator [J]. Powder Technology, 2005, 153(3): 166-175.
[5] MONDELAERS D, VANHOYLAND G, van den RUL H, HAEN J D, van BAEL M K, MULLENS J, van POUCKE L C. Synthesis of ZnO nanopowder via an aqueous acetate-citrate gelation method [J]. Materials Research Bulletin, 2002, 37(5): 901-914.
[6] HONG R Y, PAN T T, QIAN J Z, LI H Z. Synthesis and surface modification of ZnO nanoparticles [J]. Chemical Engineering Journal, 2006, 119(2/3): 71-81.
[7] WANG C L, MAO B D, WANG E B, KANG Z H, TIAN C G. Solution synthesis of ZnO nanotubes via a template-free hydrothermal route [J]. Solid State Communications, 2007, 141(11): 620-623.
[8] MENG X Q, ZHAO D X, SHEN D Z, ZHANG J Y, LI B H, WANG X H, FAN X W. ZnO nanorod arrays grown under different pressures and their photoluminescence properties [J]. Journal of Luminescence, 2007, 122/123: 766-769.
[9] RATABOUL F, NAYRAL C, CASANOVE M J, MAISONNAT A, CHAUDRET B. Synthesis and characterization of monodisperse zinc and zinc oxide nanoparticles from the organometallic precursor [Zn(C6H11)2] [J]. Journal of Organometallic Chemistry, 2002, 643/644: 307-312.
[10] ZHOU Z W, PENG W. M, KE S Y, DENG H. Tetrapod-shaped ZnO whisker and its composites [J]. Journal of Materials Processing Technology, 1999, 89/90: 415-418.
[11] MU?OZ-SANJOS? V, TENA-ZAERA R, MART?NEZ-TOM?S C, Z??IGA-P?REZ J, HASSANI S, TRIBOULET R. A new approach to the growth of ZnO by vapour transport [J]. Physica Status Solidi (C), 2005, 2(3): 1106-1114.
[12] ZHENG W W, GUO F, QIAN Y T. Growth of bulk ZnO single crystals via a novel hydrothermal oxidative pressure-relief route [J]. Advanced Functional Materials, 2005, 15(2): 331-335.
[13] LI M, LIU X L, CUI D L, XU H Y, JIANG M H. Preparation of ZnO bulk porous nanosolids of different pore diameters by a novel solvothermal hot press (STHP) method [J]. Materials Research Bulletin, 2006, 41(7): 1259-1265.
[14] VOROBYOVA S A, LESNIKOVICH A I, MUSHNSKII V V. Interphase synthesis and characterization of zinc oxide [J]. Materials Letters, 2004, 58(6): 863-866.
[15] HUGHES W L, WANG Z L. Formation of piezoelectric single- crystal nanorings and nanobows [J]. Journal of the American Chemical Society, 2004, 126(21): 6703-6709.
[16] GAO P X, MAI W J, WANG Z L. Superelasticity and nanofracture mechanics of ZnO nanohelices [J]. Nano Letters, 2006, 6(11): 2536-2543.
[17] LAO C S, LIU J, GAO P X, ZHANG L Y, DAVIDOVIC D, TUMMALA R, WANG Z L. ZnO nanobelt/nanowire Schottky diodes formed by dielectrophoresis alignment across Au electrodes [J]. Nano Letters, 2006, 6(2): 263-266.
[18] KALE R B, HSU Y J, LIN Y F, LU S Y. Synthesis of stoichiometric flowerlike ZnO nanorods with hundred per cent morphological yield [J]. Solid State Communications, 2007, 142(5): 302-305.
[19] WAHAB R, ANSARI S G, KIM Y S, SEO H K, KIM G S, KHANG G, SHIN H S. Low temperature solution synthesis and characterization of ZnO nano-flowers [J]. Materials Research Bulletin, 2007, 42(9): 1640-1648.
[20] GENG B Y, LIU X W, WEI X W, WANG S W. Large-scale synthesis of single-crystalline ZnO nanotubes based on polymer-inducement [J]. Materials Research Bulletin, 2006, 41(10): 1979-1983.
[21] REN X L, HAN D, CHEN D, TANG F Q. Large-scale synthesis of hexagonal cone-shaped ZnO nanoparticles with a simple route and their application to photocatalytic degradation [J]. Materials Research Bulletin, 2007, 42(5): 807-813.
[22] QI H X, LI Q S, WANG C F, ZHANG L C, LV L. Effects of oxygen pressure on n-ZnO/p-Si heterojunctions fabricated using pulsed laser deposition [J]. Vacuum, 2007, 81(8): 943-946.
[23] SINGH P, CHAWLA A K, KAUR D, CHANDRA R. Effect of oxygen partial pressure on the structural and optical properties of sputter deposited ZnO nanocrystalline thin films [J]. Materials Letters, 2007, 61(10): 2050-2053.
[24] HU X L, ZHU Y J, WANG S W. Sonochemical and microwave- assisted synthesis of linked single-crystalline ZnO rods [J]. Materials Chemistry and Physics, 2004, 88(2/3): 421-426.
[25] ZHANG J W, WANG W, ZHU P L, CHEN J M, ZHANG Z J, WU Z S. Synthesis of small diameter ZnO nanorods via refluxing route in alcohol–water mixing solution containing zinc salt and urea [J]. Materials Letters, 2007, 61(2): 592-594.
(Edited by HE Yun-bin)
Foundation item: Project(20876100) supported by the National Natural Science Foundation of China; Project(20090451176) supported by the China Post- doctoral Science Foundation; Project(2009CB219904) supported by the National Basic Research Program of China; Projects(YJS0917, SG0978) supported by the Commission of Science and Technology of Suzhou Municipality; Project(11C26223204581) supported by the Ministry of Science and Technology; Project(BK2011328) supported by the Natural Science Foundation of Jiangsu Province, China
Received date: 2011-02-21; Accepted date: 2011-05-16
Corresponding author: HONG Ruo-yu, Professor, PhD; Tel: +86-512-65882057; E-mail: rhong@suda.edu.cn