J. Cent. South Univ. (2016) 23: 2214-2222
DOI: 10.1007/s11771-016-3279-6
Germanium separation and purification by leaching and precipitation
Saeid Bayat, Sajjad Aghazadeh, Mohammad Noaparast, Mahdi Gharabaghi, Behrooz Taheri
School of Mining Engineering, College of Engineering, University of Tehran, Iran
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: In this research work, extraction and purification of germanium from zinc leach residues (ZLR) were investigated. The results of ICP, XRF, and atomic adsorption spectroscopy (AAS) tests show that contents of germanium, iron, lead, and zinc within the leaching residue were 105×10-6, 3.53%, 10.35%, and 8.8%, respectively. XRD results indicate that the main minerals were in different forms of sulfates (CaSO4·2H2O, PbSO4 and ZnSO4·6H2O), silicate (SiO2), and oxide (Fe2O3). Dissolution of leaching filter cake was carried out using 5 parameters and each in 4 levels (acid concentration, temperature, time, liquid-to-solid ratio, and stirring speed) by Taguchi method (L16), and then optimization of the effective parameters by response surface method. Under optimum conditions, zinc and germanium dissolution efficiencies were 88.71% and 8%, respectively. Leaching tests with sulfuric acid (added di-ammonium oxalate monohydrate) and hydrochloric acid (HCl) on the residues obtained from previous-stage sulfuric acid dissolution, yielded germanium and iron recoveries of 83%, 88%, 40%, and 90%, respectively. Thus, leaching experiment with sulfuric acid (added di-ammonium oxalate monohydrate) was superior to that with hydrochloric acid due to high and low extraction amounts of germanium and iron, respectively. Precipitation experiments revealed that germanium purification with tannic acid presented a better result compared to sodium hydroxide and ammonia. Under optimum conditions, contents of germanium and iron in the solution after precipitation were 0.1505% and 14.7% with precipitation yields of 91% and 52%, respectively.
Key words: experimental design; di-ammonium oxalate monohydrate; germanium; leaching, tannic acid
1 Introduction
Zinc production plants are commonly processed through hydrometallurgical methods to extract and purify zinc. Zinc plant residues (ZPR) produced from zinc hydrometallurgical plant, include many valuable elements such as lead, iron, germanium, nickel, cobalt, and cadmium [1]. Due to the lack of proper technology for recovery of these valuable metals, residues produced during zinc production in the plants were stored to reuse them. However, storing such materials could result in serious environmental issues in the plant atmosphere and outside as well [1].
In 1940, ZISCHKAU and WOODBRIDGE [2] proposed a procedure for germanium separation produced from a cadmium extraction process using concentrated sulfuric acid along with SO2 injection to dissolve germanium-bearing residues. LEBLEO et al [3] suggested that to achieve oxide germanium with purity more than 99.99% from zinc ores, the ore should be subjected to non-oxide media to reach germanium tetrachloride when dissolved with hydrochloric acid. Then, germanium tetrachloride was converted to pure germanium oxide after passing several separation stages in distillation columns and impurity removals [3]. MENENDEZ et al [4] used an organic solution of a tertiary amine from solution diluted by the addition of tartaric acid then stripping it with sodium hydroxide as pure germanium [4].
Germanium separation from sulfate solutions using hydroxamic acid HGS98 was studied in Ref. [1]. In this work, solution was employed which included significant amount of indium obtained during zinc hydrometallurgical process. According to this work, about 99% of germanium can be extracted into solution using 2% organic solution of hydroxamic acid and 5% of DE2HPA with organic-to-aqueous ratio of 1:5 in 5 min. Stripping of germanium succeeded with 98% efficiency using NH4F (2 mol/L) with organic-to-aqueous ratio of 1:1 in 15 min [5]. LIANG et al [6] evaluated the effective parameters causing reduction of germanium dissolution during sphalerite pressure oxidation leaching. In this work, the effects of time and temperature on the three processes including dissolved germanium hydrolysis, simultaneous precipitation with silica gel, and simultaneous precipitation iron oxide which are normally considered the three effective parameters on the reduction of germanium dissolution in the solution, were investigated. Results showed that simultaneous precipitation with silica gel was the main and most influential parameter on the germanium dissolution. Also,precipitation with iron oxide and germanium hydrolysis, respectively, were found to be the next effective parameters on the dissolution of germanium [6].
In another attempt by LIANG et al [7], under optimum conditions using trioctylamine as germanium extractant, germanium extraction rate of 97% was obtained. NaOH was used as stripping agent and striping rate achieved 95% for germanium [7]. From another point of view, in 2014, SOYLAK and YIGIT [8] investigated the possibility of concentration and separation of germanium on polysulfone membrane filter in pH=4. In this experiment, germanium was then eluted with phosphoric acid from the membrane [8].
In the current work, germanium extraction and purification from hydrometallurgical zinc plant was investigated. Optimization of the most effective parameters on the germanium dissolution and purification germanium solution was also studied.
2 Material and methods
2.1 Sample preparation
The required samples used for this work experiments were taken from filter cake produced from dissolution of zinc-bearing ore (zinc leach residues) in calcimine zinc company. Due to the aggregate and rigid form of filter cake, comminution and homogenization of samples for preparing representative samples were performed. About 30 kg of sample was taken from leaching filter cake of zinc plant stockpile. Then, sample was ground using ball mill with residence time of 8 min to reach particle size 100% finer than 2.38 mm. Finally, 1 kg of sample was prepared in each run to conduct leaching experiments.
2.2 Experiments
2.2.1 Dissolution of ZLR
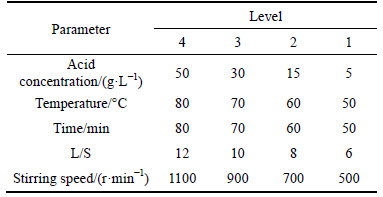
The aim of these studies was to find the optimum leaching condition of filter cake to achieve the maximum amount of zinc dissolution and its removal from filter cake along with a minimum amount of germanium dissolution. Taguchi method (Orthogonal L16) was used to evaluate effective parameters on the filter cake to remove zinc and purify germanium in the waste residue. Then, by applying response surface methodology effective parameters were optimized. Table 1 illustrates studied parameters and their levels.
2.2.2 Dissolution of residues obtained from ZLR dissolution
In this series of experiment, the effective parameters on the dissolution of residues obtained from ZLR dissolution from previous stage were examined in two different acid media (sulfuric acid and hydrochloric acid). Optimum value for each parameter for the next experiment was determined based on the previous optimum value (classic method).
Table 1 Experiment condition for selected parameters and their levels
In the leaching experiments, 150 mL of acid with calculated amount of concentration was added to 500 mL Beaker. Solution temperature was controlled by heater and stirring speed was fixed at 600 r/min. After reaching supposed temperature, germanium cake obtained from dissolution of ZLR was charged. Charged weight of cake was dependent on S/L ratio (the ratio of added solid to solution) and could be changed. To prevent solution inside leaching tank from possible evaporation, foil with aluminum material was used. At the end of each test, obtained product was filtered by vacuum filter and then dried. Finally, each product (solid residues and solution) was analyzed. The same process was applied for hydrochloric acid leaching.
2.2.3 Precipitation
In the precipitation experiments, the possibility of germanium precipitation through two different methods including precipitation by pH adjustment, and Tannic acid was investigated. Optimum value for each parameter was determined based on the obtained optimum value (classic method) to be used in the next experiment.
1) Precipitation by pH adjustment
For precipitation, obtained solution from acidic leaching was exposed to alkaline dilute solution of ammonia and sodium hydroxide agents. For all tests, 100 mL of obtained solution was added into a beaker with the capacity of 250 mL. Temperature was controlled with heater and stirring speed was fixed at 500 r/min with magnet during all the experiment. After reaching to the proposed temperature, alkaline agent (NaOH or NH3) was added to solution by using dropper until appropriate pH value achieved. Leaching experiment was continued for 1 h for all tests after assumed pH value achieved. Continually, residence time of 5 h was set for precipitation after the end of each leaching time, then precipitation was cooled, filtered, dried, weighted, and finally was sent to laboratory for the chemistry analysis to measure germanium and iron contents.
2) Precipitation by tannic acid
These experiments were preceded for precipitation by pH adjustment; however, tests were conducted using ammonia as pH regulator and temperature was set at 90 °C. Accordingly, certain amount of tannic acid was weighed and then added into 10 mL distilled water at temperature of 50 °C.
3 Sample characterization
3.1 Sample mineralogy
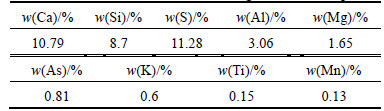
Atomic absorption spectrometry (AAS) was used for determination of chemical elements in leaching filter cake. Table 2 lists that lead, zinc, and iron were the most dominant elements and constitute 10.35%, 8.80%, and 3.53% in the leaching filter cake, respectively. Based on XRF results listed in Table 3, calcium, silicon, and sulfur were considered main impurities. Also, ICP method revealed that germanium constitutes 105×10-6 of leaching filter cake.
Table 2 Atomic absorption spectrometry result for zinc, lead, and iron in leaching filter cake sample
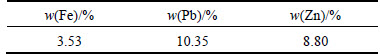
Table 3 Obtained XRF result for leaching filter cake sample
3.2 Phase identification for leaching filter cake
In order to examine different phases of leaching filter cake, XRD test was used and showed that the main phases included gypsum (CaSO4·2H2O), lead sulfate (PbSO4), zinc sulfate (ZnSO4·6H2O), silicate (SiO2), and iron oxide (Fe2O3).
4 Results and discussion
4.1 Dissolution of ZLR
In order to find the most effective parameters on the dissolution efficiency of germanium and zinc in leaching filter cake of ZPR (zinc plant residues), the Taguchi method (orthogonal L16) was initiated. The obtained results from analysis of variance (ANOVA) for each responses (germanium and zinc) are listed in Tables 4 and 5.
As listed in Table 4, acid concentration, leaching time and temperature played a key role in the dissolution of zinc; however, due to small value of F for L/S and stirring speed they did not significantly affect zinc dissolution. Considering F value, L/S ratio was considered to be ignorable on the proposed model for zinc dissolution efficiency.
According to Table 5 for germanium dissolution, parameters of L/S ratio, acid concentration, and temperature were found to be the most effective parameters, respectively. But leaching time due to small value of F was considered to be an ignorable factor on proposed model for germanium dissolution efficiency. Moreover, comparing calculated Fisher value (F05/0= 9.276) with F value for each effective parameters, it can be postulated that proposed model based on the effective parameters was validated.
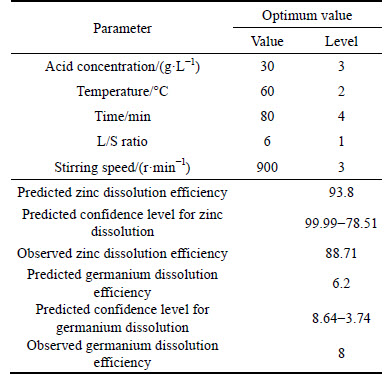
1) In order to optimize effective parameters on germanium and zinc dissolution, response surface methodology was used. The aim of this optimization was to minimize germanium dissolution while maximizing zinc dissolution. The results are given in Table 6.
As it can be seen in Table 6, observed dissolution efficiency of zinc under optimum condition in the laboratory was 88.71% and predicted value by design software through RSM was 93.8%, while these values for germanium were 8% and 6.2%, respectively. These results indicated that dissolution and transferring of zinc from leaching filter cake into solution and also purification of germanium in the obtained residue which were the aim of experiments, were successfully achieved.
Table 4 Analysis of variance results for zinc dissolution response
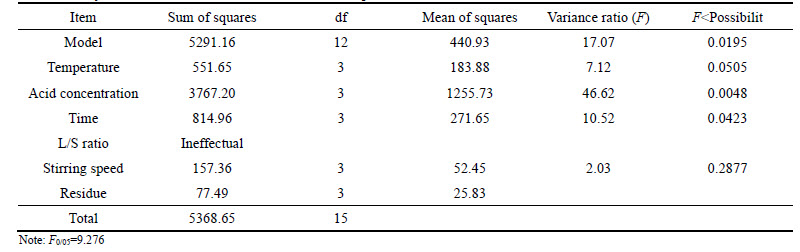
Table 5 Analysis of variance for germanium dissolution response

Table 6 Optimum conditions for germanium and zinc dissolution by RSM
4.2 Dissolution of residues obtained from ZLR dissolution
4.2.1 Sulfuric acid leaching
1) Effects of acid concentration on germanium and iron dissolution
As it is shown in Fig. 1, increasing sulfuric acid concentration had a significant effect on iron dissolution as in acid concentration of 150 g/L, about 75% iron was dissolved into solution. In addition, increasing sulfuric acid concentration up to 150 g/L tends to improve the dissolution efficiency of germanium. That is because the increasing sulfuric acid concentration, intensifies germanic acid formation speed, which leads to dissolution rate of geranium in the solution [9]. However, for sulfuric acid concentrations more than 150 g/L dissolution efficiency decreases. This may be due to this fact that with increasing sulfuric acid concentration, dissolved germanium in the solution hydrolyzes into germanium oxide and because germanium oxides in acid media are insoluble, they will enter leaching waste residues [10-11].
2) Effect of temperature on germanium and iron dissolution
Temperature is one of the most effective parameters in dissolution reaction progress. For this series of experiment, sulfuric acid concentration was chosen at 150 mg/L (optimum value) as it was obtained from previous experiment. Based on Fig. 2, increasing temperature has remarkably improved the dissolution efficiency of germanium and iron. Temperature increase from 50 to 90 °C causes the recovery of germanium and iron dissolution efficiency to increase 23% (from 42% to 65%) and 30% (from 47% to 77%). Temperature influences the rates of reaction through kinetic energy, such that high temperatures increase the kinetic energy of reacting molecules, therefore, causing frequent collisions, which form products (Ge and Fe) faster.
3) Effect of leaching time on germanium and iron dissolution
Considering plots of obtained results in Fig. 3, increasing leaching time from 0.5 to 3.5 h causes the germanium and iron dissolution efficiency to increase 30% and 40%, respectively. However, further increase of leaching time (more than 3.5 h) leads to a loss in both germanium and iron dissolution. Although, the reason is dissolution reaction for germanium and iron reaches equilibrium state after a long leaching time, due to their silicate forms which are insoluble in acid media, some parts of germanium and iron do not dissolve in the solution, causing a drop in dissolution efficiency [11-12].
4) Effect of pulp density on germanium and iron dissolution
Increasing pulp density has significantly decreased dissolution efficiency for both germanium and iron (Fig. 4). The reason for this phenomenon refers toincrease in reaction area of solid, especially in dilute solution, while the amount of acid concentration has not been changed. Moreover, for small values of pulp density (larger L/S ratio), dissolution efficiencies for both germanium and iron are lower when it reaches L/S ratio of 10. This may be attributed to small amount of solid particles available per mole of acid whereby some amount of acid remains in the solution without reaction with germanium and iron. Therefore, pulp density of 1/8 (L/S ratio) was considered optimum vale for this experiment.
Table 7 Mass balance results for leaching filter cake dissolution under optimum condition

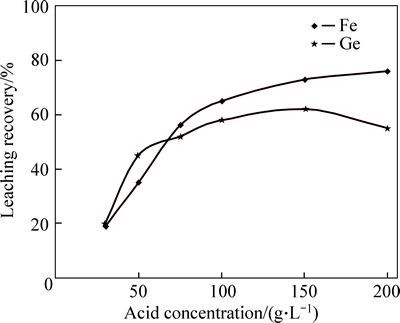
Fig. 1 Effect of sulfuric acid concentration on germanium and iron yield (Temperature=85/°C, L/S=1/7, Time=3 h, and stirring speed=600 r/min)
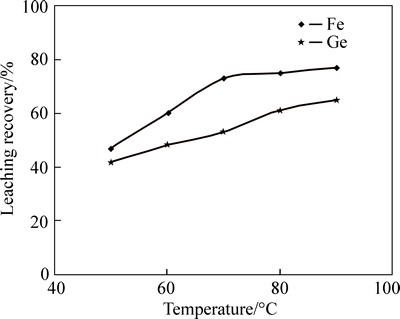
Fig. 2 Effect of temperature variation on germanium and iron yield (Sulfuric acid concentration=150 g/L, L/S=1/7, Time=3 h, and stirring speed=600 r/min)
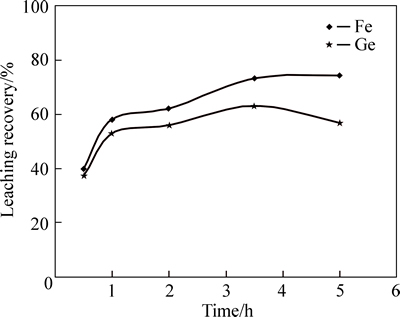
Fig. 3 Effect of time variation on germanium and iron yield (Sulfuric acid concentration=150 g/L, Temperature=90 °C, L/S=1/7, and stirring speed=600 r/min)
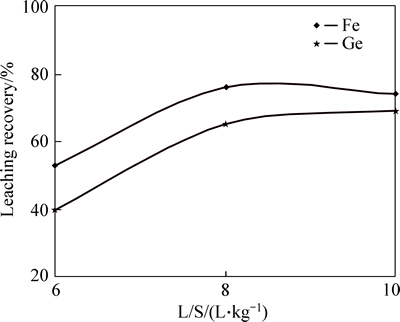
Fig. 4 Effect of density variation on germanium and iron yield (Sulfuric acid concentration=150 g/L, Temperature=90 °C, Time=3.5 h, and stirring speed=600 r/min)
5) Effect of di-ammonium oxalate monohydrate on germanium and iron dissolution
Germanium and iron dissolution efficiencies obtained from previous experiments under optimum conditions for sulfuric acid concentration of 150 g/L, temperature of 90 °C, leaching time of 3.5 h, and pulp density of 1/8 (kg·L-1) were 65% and 74% , respectively, without using di-ammonium oxalate monohydrate. Therefore, a large amount of germanium does not dissolved and remains in waste residues. This necessitates using a complex agent for improving germanium dissolution. Di-ammonium oxalate monohydrate was used as complex agent of germanium (Fig. 5).
Increasing di-ammonium oxalate monohydrate (prepared from Merck Company) up to 4 g causes the dissolution efficiency of germanium to improve.This might be because of forming di-ammonium oxalate monohydrate complex soluble in acid media(H2Ge(C2O5)3) in the presence of di-ammonium oxalate monohydrate and also more dissolution of germanium oxide insoluble in acid media [10]. For di-ammonium oxalate monohydrate more than 4 g in the solution, the dissolution efficiency tends to decrease. For large amount of di-ammonium oxalate monohydrate concentration, oxalate formation and then its adsorption on solid particles prevents minerals from further dissolution [13]. Dissolution efficiencies of germanium and iron for sulfuric acid leaching with di-ammonium oxalate monohydrate (optimum value of 4 g) under optimum condition were obtained 83% and 88%, respectively.
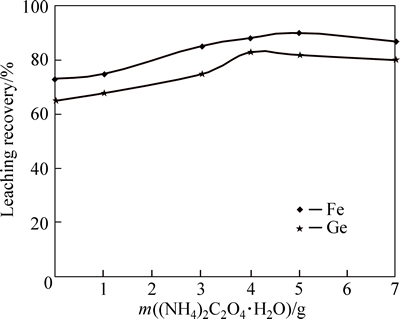
Fig. 5 Effect of di-ammonium oxalate monohydrate amount on germanium and iron yield
4.2.2 Optimization
After leaching waste residues (obtained from dissolution of ZLR) by hydrochloric acid under optimum condition, a solution was obtained including 9.1×10-6 germanium, 6.1 g/L iron, and 5.17 g/L lead. By applying a residence time of 8 h for obtained solution to remove time-dependent impurities (such as lead) to precipitate, a final solution with 9.3×10-6 germanium, 5.8 g/L iron, and 2.5 g/L lead was achieved. Under optimum condition for sulfuric acid leaching to obtain a maximum dissolution of germanium and iron, a solution with 19×10-6 germanium and 5.22 g/L iron was achieved. Therefore, according to obtained results, leaching by sulfuric acid was considered the most appropriate method for germanium extraction from waste residues (obtained from ZLR dissolution). Results are given in the Table 8.
Table 8 Mass balance result for sulfuric leaching of waste residues under optimum condition
4.3 Precipitation tests
4.3.1 Precipitation by pH adjustment
1) Effect of pH on germanium and iron by sodium hydroxide
As shown in Fig. 6, increasing pH significantly increased iron and specifically germanium precipitation yields. The reason may be that by increasing pH, the formation possibility of some complexes such as (Fe(OH)3), (xFe(OH)3·yH2O·zFe3+), and (xFe2O3·yH2O·zFe3+) in the solution tends to increase. As a result, adsorption of germanium anionic complexes such as HGeO3- or GeO32- takes place which causes germanium to precipitate in the solution [14]. In addition, for pH increase more than 5.5, it shows that there is no sharp increase in precipitation yield and even could bring more impurities into solution.

Fig. 6 pH adjustment by sodium hydroxide and its effect on germanium and iron precipitation (Temperature=85 °C, stirring speed=400 r/min)
2) Effect of pH on germanium and iron precipitation yield by ammonia
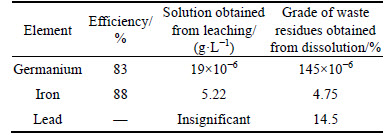
Figure 7 presents that increasing of pH (ammonia as pH adjusting) from 2.5 to 5.5 causes the precipitation yields of germanium and iron to increase from 18% to 55% and 33% to 99%, respectively. When pH of solution reaches 5.5, precipitation of iron almost is complete; however, a large amount of germanium did not adsorb on deposited iron and remained in the solution. Moreover, increasing pH more than 5.5 did not improve germanium precipitation and could precipitate some unwanted impurities.
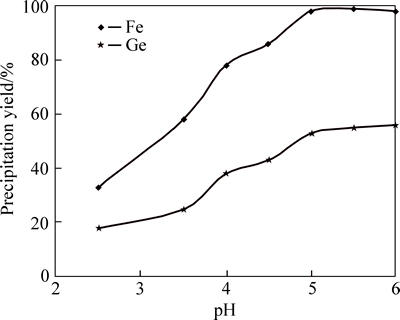
Fig. 7 pH adjustment by ammonia and its effect on germanium and iron precipitation
Comparing obtained results for both ammonia and sodium hydroxide as pH adjusting agent to precipitate germanium and iron from solution, under optimum condition for ammonia (Temperature 85 °C, pH 5.5) germanium and iron precipitation yields were 55% and 99%, respectively; however, considering the same condition for sodium hydroxide, germanium and iron precipitation yields were 85% and 97%, respectively. Therefore, sodium hydroxide presents a better precipitation result for both germanium and iron compared to ammonia.
3) Effect of temperature on germanium and iron precipitation yields with sodium hydroxide
Figure 8 shows that increasing temperature from 50 °C to 80 °C increases germanium and iron precipitation yields from 55% to 85% and 75% to 98%, respectively. For temperature higher than 80 °C, precipitation yield tends to decrease. This may be because by further increase in temperature, insoluble iron hydroxide deposits redissolve in the solution [15]. Thus, germanium precipitation has a direct relationship with iron precipitation, by decreasing iron precipitation, subsequently germanium precipitation tends to decrease.
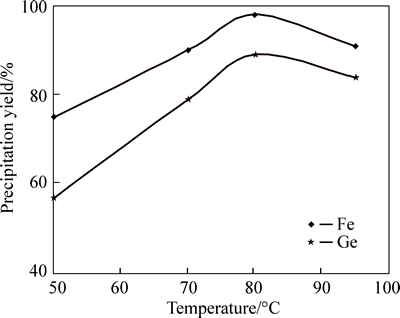
Fig. 8 Effect of temperature variation on germanium and iron precipitation (Temperature=80 °C, stirring speed=400 r/min, pH=5.5 by NaOH)
The obtained results from atomic adsorption spectrometry (AAS) under optimum condition for germanium and iron precipitation by sodium hydroxide are presented in Table 9.
4.3.2 Precipitation by tannic acid
1) Effect of pH on germanium precipitation yield by tannic acid
According to Fig. 9, when pH of solution reaches 1.5, germanium and iron precipitation yields ware 85%and 34%, respectively. In other words, at pH=1.5 germanium precipitation is completed and there is no need for pH increase because it causes more iron precipitation.
Table 9 Sodium hydroxide precipitation results for germanium and iron

Fig. 9 Effect of pH variation on germanium and iron precipitation (Temperature=80 °C, stirring speed=400 r/min)
Dilute ammonia was used for pH increase during precipitation with tannic acid (purity of 99%, produced by Sigma-Aldrich Company). Based on some studies, the presence of ammonium ion in the solution plays a key role during germanium precipitation by tannic acid [13, 16]. Therefore, it could be postulated to increase pH by adding ammonia into solution.
2) Effect of tannic acid amount on germanium precipitation yield
In order to examine the effect of tannic acid amount on germanium precipitation yield in the solution, 0.01, 0.05, 0.1, 0.15, 0.2, 0.3, and 0.5 g tannic acid were added to 100 mL of solution. Results are shown in Fig. 10.
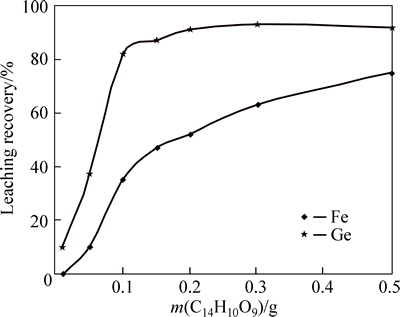
Fig. 10 Effect of tannic acid amount on germanium and iron precipitation
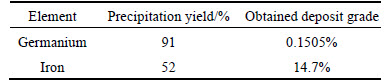
Figure 10 presents that tannic acid increase from 0.1 to 0.2 g in the solution causes germanium and iron precipitation yields increase from 10% to 91% and 0 to 52%, respectively. Tannic increase by more than 0.2 g does not affect noticeably germanium precipitation yield; however, it can precipitate more iron from the solution which is not favorite. Considering optimum condition when pH and tannic acid amounts were 1.5 and 0.2 g in 100 mL solution, respectively, results obtained by atomic adsorption spectrometry (AAS) are illustrated in Table 10. Figure 11 describes the whole process for germanium purification.
Table 10 Tannic acid precipitation results for germanium and iron
5 Conclusions
In this work, Taguchi and response surface methodology were used to first determine the most effective parameters and then optimize them in dissolution experiment of ZLR (zinc leaching residues) to remove zinc and purify germanium in the wasteresidue obtained from dissolution. The obtained results showed that the maximum zinc dissolution efficiency and the minimum germanium dissolution efficiency of 88.71% and 8% can be reached under optimum condition as sulfuric acid concentration 30 g/L, temperature 60, leaching time 80 min, L/S ratio 6, and stirring speed 900 r/min.
Extraction of germanium and iron from waste residues obtained from ZLR dissolution, by sulfuric and hydrochloric acid was investigated. In sulfuric acid media, the maximum dissolution efficiencies of germanium and iron were obtained 83% and 88% under optimum condition as sulfuric acid concentration 150 g/L, temperature 90 °C, di-ammonium oxalate monohydrate 4 g within 150 mL solution, S/L ratio of 1/8, stirring speed 600 r/min, and leaching time 3.5 h. In hydrochloric acid, the maximum germanium dissolution 40% was obtained under optimum condition for hydrochloric acid concentration 3 mol, temperature 90 °C, S/L ratio 1/7, stirring speed 600 r/min, and leaching time 1 h. In addition, iron and lead dissolution efficiencies were 90% and 25% for hydrochloric acid experiment. Considering the obtained results for both sulfuric and hydrochloric acid, leaching with sulfuric acid was chosen as the best method for germanium extraction from residues obtained from ZLR dissolution. Leaching under optimum conditions to obtain a maximum dissolution efficiency of germanium and iron, obtained solution contained 19×10-6 of germanium and 5.22 g/L of iron. In addition, using di-ammonium oxalate monohydrate and obtained results indicate that it acts as increasing agent of germanium dissolution rate in the solution.
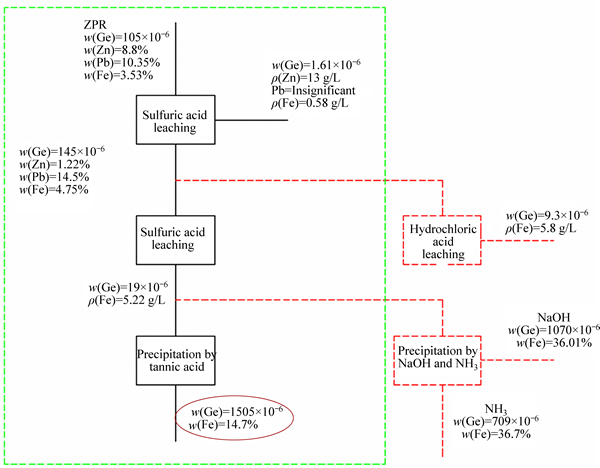
Fig. 11 A drawn flow-sheet for germanium purification
pH adjustment for precipitation purpose by adding ammonia and sodium hydroxide was also investigated. Increasing pH from 2.5 to 5.5 by ammonia and sodium hydroxide presented iron and germanium precipitation yields of 95%, 87% (results for ammonia) and 99%, 55% (results for sodium hydroxide), respectively. Therefore, due to high precipitation yield by adding sodium hydroxide, under optimum condition, germanium and iron precipitation yields were 89% and 98%, respectively. Also, the obtained deposit contained 36.01% iron and 0.107% germanium.
For selective precipitation of germanium by tannic acid, ammonium ion was considered the most important agent for precipitation. Therefore pH adjustment was done by adding ammonia to solution. It is found that by adding 0.1 g tannic acid into solution and increasing pH up to 1.5, 85% of germanium and 34% of iron could be precipitated. Under optimum condition, germanium and iron precipitation yield of 91% and 52% were obtained by adding sodium hydroxide. Consequently, obtained deposit contained 14.7% of iron and 0.1505% of germanium.
Acknowledgement
This research was supported by Zanjan Calcimin Zinc Company. The authors would like to appreciate them for their contribution and help during this project.
References
[1] ZHOU Z A, GUANG C H, GAN H X, YANG T Z, LIN C H. Ge and Cu recovery from precipitating vitriol supernatant in zinc plant [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1506-1511.
[2] ZISCHKAU C, WOODBRIDGE N J. Separation of germanium: US, Patent 2249341 [P]. 1940.
[3] LEBLEO A, FOSSI P, DEMARTHE J M. Process for the recovery and purification of germanium from zinc ore: US, Patent 4090871 [P]. 1978.
[4] MENENDEZ F J S, MENENDEZ M S, CUADRA HERRERA A D L, TAMARGO F A, LORENZO L P, VALCARCEL M R, FERNANDEZ V A. Process for the recovery of germanium from solution that contain it: US, Patent 4886648 [P]. 1989.
[5] TANG S F, ZHOU C S, JIANG X Y, ZHAO C L. Extraction separation of germanium with hydroxamic acid HGS98 [J]. Journal of Central South University of Technology, 2000, 7(1): 40-42.
[6] LIANG D, WANG J, WANG Y. Difference in dissolution between germanium and zinc during the oxidative pressure leaching of sphalerite [J]. Hydrometallurgy, 2009, 95(1): 5-7.
[7] LIANG J, FAN L, XU K, HUANG Y. Study on extracting of germanium with trioctylamine [J]. Energy Procedia, 2012, 17: 1965-1973.
[8] SOYLAK M, YIGIT S. Preconcentration–separation of germanium at ultra trace levels on polysulfone membrane filter and its determination by spectrophotometry [J]. Journal of Industrial and Engineering Chemistry, 2015, 24: 322-325.
[9] WOOD S A, SAMSON I M. The aqueous geochemistry of gallium, germanium, indium and scandium [J]. Ore Geology Reviews, 2006, 28(1): 57-102.
[10] HE J, TANG M T, LIU Z Q, YANG S H, YAO W Y. Concentrating Ge in zinc hydrometallurgical process with hot acid leaching-halotrichite method [J]. Journal of Central South University of Technology, 2003, 10(4): 307-312.
[11] HARBUCK D D. Gallium and germanium recovery from domestic sources [M]. Washington D. C. USA: US Department of the Interior, Bureau of Mines, 1992.
[12] KUL M, TOPKAYA Y. Recovery of germanium and other valuable metals from zinc plant residues [J]. Hydrometallurgy, 2008, 92(3): 87-94.
[13] DU F, LI J, LI X, ZHANG Z. Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid [J]. Ultrasonics Sonochemistry, 2011, 18(1): 389-393.
[14] NUSEN S, ZHU Z, CHAIRUANGSRI T, CHENG C Y. Recovery of germanium from synthetic leach solution of zinc refinery residues by synergistic solvent extraction using LIX 63 and Ionquest 801 [J]. Hydrometallurgy, 2015, 151: 122-132.
[15] VIROLAINEN S, HEINONEN J, PAATERO E. Selective recovery of germanium with N-methylglucamine functional resin from sulfate solutions [J]. Separation and Purification Technology, 2013, 104: 193-199.
[16] ARROYO F, FONT O,  C, QUEROL X, CHIMENOS J, ZEEGERS H. Germanium and gallium extraction from gasification fly ash: Optimisation for up-scaling a recovery process [C]// World of Coal Ash Conference. Lexington, KY, USA, 2009. http://www. flyash. info.
C, QUEROL X, CHIMENOS J, ZEEGERS H. Germanium and gallium extraction from gasification fly ash: Optimisation for up-scaling a recovery process [C]// World of Coal Ash Conference. Lexington, KY, USA, 2009. http://www. flyash. info.
(Edited by YANG Hua)
Received date: 2015-07-21; Accepted date: 2016-01-05
Corresponding author: Mahdi Gharabaghi, PhD; Tel: +98-21-82084556; E-mail: gharabaghi@ut.ac.ir, m.gharabaghi@gmail.com