
Modified multi-walled carbon nanotubes with nano-europium oxide
CHEN Chuan-sheng(陈传盛)1, 2, CHEN Xiao-hua(陈小华)1, YI Bin(易 斌)1,
ZHANG Guo-bin(张国斌)3, LI Fu-jin(李富进)1, LUO Hui-shan(罗慧珊)1
1. College of Materials Science and Engineering, Changsha University of Science and Technology,Changsha 410076, China;
2. State Key Laboratory for Powder Metallurgy, Central South University, Changsha 410083, China;
3. National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230026, China
Received 15 July 2007; accepted 10 September 2007
Abstract: In order to improve optical property of the multi-walled carbon nanotubes (MWNTs), MWNTs were decorated with europium oxide (Eu2O3) nanoparticles by using co-deposition method. The MWNTs/Eu2O3 composites were examined by XRD, scanning electron microscopy, transmission electron microscopy, and VUV-Vis Luminescence spectroscopy and citric acid (CA) molecules were introduced onto the surface of MWNTs. The results show that there are many oxygenated functional groups on the surface of the MWNTs after the treatment of mixture acid, such as carboxy, hydroxl, carbony and amidocyanogen. The results of electron microscopy illuminate that the MWNTs are coated by nano-europium oxide after annealed at 750℃. The MWNTs/Eu2O3 composite emits much strong red light at about 610 nm under UV excitation.
Key words: carbon nanotubes; nano-europium oxide; fluorescence property
1 Introduction
The combination of carbon nanotubes (CNTs) and nano-particles can both improve the dispersion of CNTs in composite and enhance the compatibility between CNTs and based material, therefore improving the properties of composite[1-7]. Furthermore, combined unique properties of CNTs and nanoparticles make the nanoparticle-nanotube composite structure have advanced applications[8-13], including nanoelectronics, chemical sensors, biosensors, catalysis, fuel cells, etc.
MWNTs have a hollow structure with inner diameters of the order of 100 nm, which is an ideal geometry for drug transport and delivery. However, MWNTs have only been observed to exhibit weak infrared emissions. In order to improve their infrared emissions, MWNTs must be functionalized with spectroscopically characteristic fluorescent dyes. The unique electronic, optical, and chemical properties of the rare earth oxides make them useful in a variety of diverse applications such as laser materials, phosphors, and catalysis. The luminescence of Eu3+ is particularly interesting because the major emission band is centered near 612 nm (red), which is one of the three primary colors. In addition, Eu2O3 is one of the most important oxide phosphors and has been studied extensively[14-16]. The assembly of Eu2O3 on the surface of MWNTs can improve the luminescence property of MWNTs, and it can be used in luminescence material and the fields of cancer diagnosis and therapy. SUN et al[17] gained MWNTs/Eu2O3 composite of red luminescence. FU et al[18] reported that the luminescence property of MWNTs was improved by coating of Eu2O3. SHI et al[19] reported that the MWNTs exhibited luminescent emission in the visible-light range by depositing Eu2O3-doped Y2O3 nanophosphors onto the outside surfaces of MWNTs. These results indicate that surface-functionalized CNTs have wide applications in both novel optical devices and cancer diagnosis and treatment.
Although the Eu2O3 nanoparticles have been assembled on the surface of MWNTs by using different methods, the mechanism of assembled nanoparticles on the MWNTs and the properties of composite nanotubes need to be further investigated. In this work, the surfacefunctionalization of MWNTs was studied, and the fluorescence property of MWNTs/Eu2O3 composite was examined by VUV-Vis luminescence spectroscopy.
2 Experimental
2.1 Functionalization of MWNTs
The MWNTs used in this work were produced by catalytic decomposition of acetylene[20]. The products were oxidized by immersing in mixture acid and refluxed for 2 h at boiling, and then suspended and refluxed in HCl solution for 2 h at boiling. In order to further functionalize MWNTs, the oxidized MWNTs were further refluxed in concentrated ammonia[11].
2.2 Modification of MWNTs with citric acid
The aminated MWNTs and citric acid (CA) were mixed and sonicated in de-ionized water for 30 min. Sulfuric acid (10 mL) was dropped into the suspension of MWNTs, which was then stirred and refluxed at 80 ℃. The reaction mixture was cooled to ambient temperature, then filtered and washed with deionized water. The black precipitate was collected by filtration and dried at 60 ℃.
2.3 Eu2O3 nanoparticle decorated MWNTs
Europium oxide (99.99%) was dissolved in nitric acid at 80 ℃ under continuous stirring, and then the mixed solution was heated in vacuum evaporator for evaporating redundant nitric acid. The modified MWNTs with CA were added into the europium nitrate solution, and the mixture was vibrated for 1 h. The oxalic acid and sodium hydroxide were dissolved in de-ionized water, and subsequently dropped into the MWNTs mixture solution under continuous stirring. The pH value of the mixed solution was adjusted to 6 under 70 ℃. After 1 d, the precipitate was collected by filtration and dried at 80 ℃. Finally, the MWNTs/europium xalate precursor was calcined at 750 ℃ under the protection of nitrogen.
2.4 Characterization
Infrared spectrum was measured on a Jasco 300E Infrared spectrometer. X-ray diffraction (XRD) measurements were performed using Philips PW 1710 diffractometer with Cu Kα1 radiation. The observation of scanning electron microscope (SEM) was carried out with a JSM-6700F field emission scanning electron microscope. Transmission electron microscope (TEM) analyses and energy dispersive X-ray (EDX) were conducted on a JEM-3010 transmission electron microscope of high magnification. The fluorescence spectrum was recorded with VUV-Vis luminescence spectroscopy. The excitation spectrum was observed at 610 nm, and the emission spectrum was excited at 210 nm.
3 Results and discussion
3.1 Phase structure of MWNTs/Eu2O3 composite
The MWNTs/Eu2O3 samples were characterized by recording their XRD patterns, as shown in Fig.1. It is clear that the (222), (400), (440), (102) and (622) planes of Eu2O3 are observed at 2θ of 28.368?, 32.894?, 47.244?, and 56.066?, respectively. Furthermore, there are eight puny diffraction peaks at 2θ of 34.943?, 38.793?, 42.338?, 48.260?, 51.777?, 54.648?, 57.453?, and 58.743?, respectively. They are corresponded to (411), (232), (413), (611), (523), (514), (631), and (444) planes of Eu2O3, respectively, which correspond to the reported values of Eu2O3 well[21]. These results can be indexed to the cubic structure of Eu2O3.
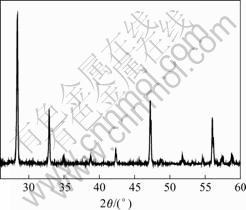
Fig.1 XRD pattern of MWNTs/Eu2O3 composite
3.2 Electron microscopy
Electron microscopic images of MWNTs/Eu2O3 heat treated at temperature of 750 ℃ are shown in Fig.2. Fig.2(a) shows the SEM image of MWNTs/Eu2O3 composite. The size of nanoparticles is 20-100 nm. Fig.2(b) shows the TEM image of MWNTs/Eu2O3 composite. It is evident that the MWNTs are modified with single nanoparticles. In order to confirm the element presented in the coating on the surface of MWNTs, EDS was carried out. The result is shown in Fig.3. This reveals the presence of Eu, O and C on the surface of the nanotubes, which confirms the existence of Eu2O3 nanoparticles on the surface of MWNTs.
3.3 Mechanism of modification
From the above results, it is concluded that the MWNTs can be coated by Eu2O3 nanoparticles after heat treatment at 750 ℃. Modified MWNTs with Eu2O3 are attributed to the surface modification of MWNTs.
After the MWNTs were oxidized by mixture acid, a

Fig.2 Image of MWNTs/Eu2O3 composite: (a) SEM; (b) TEM
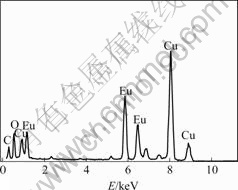
Fig.3 EDS of MWNTs/Eu2O3 composite
hydroxy group at 3 444 cm-1 and carboxyl group at 1 730 cm-1 are introduced onto the defects of the MWNTs,as shown in curve (a) of Fig.4. From the infrared spectrum of aminated MWNTs (the curve(b) of Fig.4), it is very evident that the characteristic of the V(C—N) stretching mode is observed at about 1 128 cm-1. Moreover, after the MWNTs are further treated by CA, the carbonyl stretches and the C—O bond stretches are observed at 1 714 cm-1 and 1 177 cm-1, respectively (as shown in curve (c) of Fig.4). The peak at 1 714 cm-1 originates from the carbonyl stretch of the carboxylic acid groups of CA, and the peak at 1 177 cm-1 may be attributed to the C—O bond stretches of MWNTs—O—C and C—OH units. These results illuminate that the hydroxyl group, carboxyl group, amidocyanogen group, and molecules of CA are introduced onto the surface of MWNTs by surface modification.

Fig.4 Infrared spectra of MWNTs: (a) Oxidized MWNTs; (b) Aminated MWNTs; (c) Modified MWNTs (Mass ratio of CA to MWNTs is 1?1)
On one hand, the surface modification improves the chemical activity of MWNTs. More defects are produced on the walls of MWNTs because of the treatment for MWNTs with both mixture acid and ammonia, and the interaction between MWNTs and europium oxalate precursor is improved. On the other hand, CA is an important complex, and it can act as ligand for Eu3+ to be anchored on the surface of the MWNTs. Hence, the surface modification enhances the contact between MWNTs and precursors, and makes the deposition of Eu2O3 nanoparticles facilitate on the surface of MWNTs.
3.4 Fluorescence property
Fig.5 shows the VUV-Vis luminescence of pristine MWNTs and MWNTs/Eu2O3 composite at room temperature under the same experimental conditions. The excitation spectrum of the MWNTs/Eu2O3 composite (emission at 610 nm) shows that two striking excitation peaks can be observed at 210 nm and 230 nm, with the most excitation wavelength at 210 nm, but no excitation peak is observed in the pristine MWNTs. When the excitation wavelength is 210 nm, the emission spectrum of MWNTs/Eu2O3 composites consists of six main lines at 580, 590 592, 598, 610 and 630 nm. The strongest peak is located at 610 nm corresponding to the 5D0→7F2 electric dipole transition of Eu3+. The emission peak at 580 nm is assigned to the 5D0→7F0 transition of Eu3+. The series of emission peaks at 590, 592, and 598 nm belong to 5D0→7F1 magnetic-dipole allowed transition of Eu3+. The results illustrate that the optical property of MWNTs is improved significantly by modification of Eu2O3 nanoparticles.
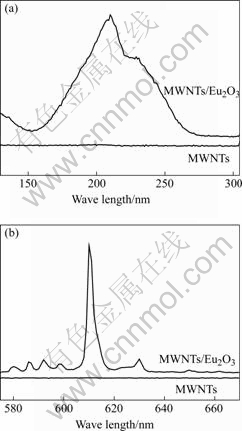
Fig.5 VUV-Vis luminescence of MWNTs and MWNTs/Eu2O3 composite: (a) Excitation spectrum (emission of 610 nm); (b) Emission spectrum (excitation at 210 nm)
4 Conclusions
1) The MWNTs are coated by Eu2O3 nanoparticle after annealed at 750 ℃.
2) The hydroxyl groups, carboxyl groups, amidocyanogen and CA molecules are introduced onto the surface of MWNTs through surface treatment.
3) The fluorescence properties of MWNTs are improved significantly through the coating of nano-europium oxide.
References
[1] CHA S I, KYUNG K T, ARSHAD S N, et al. Extraordinary strengthening effect of carbon nanotubes in metal-matrix nanocomposites processed by molecular-level mixing[J]. Adv Mater, 2005, 17(11): 1377-1381.
[2] CARE?O-MORELLI E, YANG J, COUTEAU E, et al. Carbon nanotube/ magnesium composites[J]. Phys Stat Sol, 2004, 201(8): R53-R58.
[3] HWANG G L, SHIEH Y T, HWANG K C. Efficient load transfer to polymer-grafted multi-walled carbon nanotubes in polymer composites[J]. Adv Funct Mater, 2004, 14(5): 487-491.
[4] DONG Shu-rong, ZHANG Xiao-bin. Mechanical properties of Cu based composites reinforced by carbon nanotubes[J]. The Chinese Journal of Nonferrous Metals, 1999, 9(3): 457-461. (in Chinese)
[5] CHEN C S, CHEN X H, XU L S, et al. Modification of multi-walled carbon nanotubes with fatty acid and their tribological properties as lubricant additive[J]. Carbon, 2005, 43(8): 1660-1666.
[6] WANG Lang-yun, TU Jiang-ping, YANG You-zhi, et al. Frication and wear behavior of multi-walled carbon nanotube/Cu matrix composites[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(3): 367-371. (in Chinese)
[7] CHEN C S, CHEN X H, YANG Z, et al. Effect of multi-walled carbon nanotubes as reinforced fibres on tribological behaviour of Ni-P electroless coatings[J]. Diamond and Related Materials, 2006, 15(1): 151-156.
[8] BAVASTRELLO V, CARRARA S, RAM M K, et al. Optical and electrochemical properties of poly(o-toluidine) multiwalled carbon nanotubes composite langmuir-schaefer films[J]. Langmuir, 2004, 20(3): 969-973.
[9] TAKENOBU T, TAKANO T, SHIRAISHI M, et al. Stable and controlled amphoteric doping by encapsulation of organic molecules inside carbon nanotubes[J]. Nat Mater, 2003, 2(10): 683-688.
[10] LI W Z, REN J W, DENG K, et al. Preparation and characterization of multi-walled carbon nanotube-supported platinum cathode catalysts of direct methanol fuel cells[J]. J Phys Chem B, 2003, 107(26): 6292-6299.
[11] CHEN C S, CHEN X H, YI B, et al. Zinc oxide nanoparticle decorated multi-walled carbon nanotubes and their properties[J]. Acta Materialia, 2006, 54(20): 5401-5407.
[12] YU K, ZHANG Y S, XU F, et al. Significant improvement of field emission by depositing zinc oxide nanostructures on screen-printed carbon nanotube films[J]. Appl Phys Lett, 2006, 88: 153123-1-3.
[13] ZHU Y W, HENDRY IZAAC ELIM, et al. Multiwalled carbon nanotubes beaded with ZnO nanoparticles for ultrafast nonlinear optical switching[J]. Adv Mater, 2006, 18(5): 587-592.
[14] WOLFSON1 J M, KEARNS D R. Europium as a fluorescent probe of transfer RNA structure[J]. Biochemistry, 1975, 14(7): 1436-1444.
[15] TISSUE B M. Synthesis and luminescence of lanthanide ions in nanoscale insulating hosts[J]. Chem Mater, 1998, 10(10): 2837-2845.
[16] PATRA A, SOMINSKA E, RAMESH S, et al. Sonochemical preparation and characterization of Eu2O3 and Tb2O3 doped in and coated on silica and alumina nanoparticles[J]. J Phys Chem B, 1999, 103(17): 3361-3365.
[17] SUN W X, HUANG Z P, ZHANG L, et al. Luminescence from multi-walled carbon nanotubes and the Eu(Ⅲ)/multi-walled carbon nanotube composite[J]. Carbon, 2003, 41(8): 1685-1687.
[18] FU L, LIU Z M, LIU Y Q, et al. Coating carbon nanotubes with rare earth oxide Multiwalled nanotubes[J]. Adv Mater, 2004, 16(4): 350-352.
[19] SHI D L, LIAN J, WANG W, et al. Luminescent carbon nanotubes by surface functionalization[J]. Adv Mater, 2006, 18(2): 189-193.
[20] CHEN X H, CHEN C S, CHEN Q, et al. Non-destructive purification of multi-walled carbon nanotubes produced by catalyzed CVD[J]. Materials Letter, 2002, 57(3): 734-738.
[21] DAKHEL A A. Correlated structural and optical properties of thin Eu oxide films[J]. Materials Chemistry and Physics, 2003, 80(1): 186-190.
(Edited by CHEN Can-hua)
Foundation item: Project(50372020) supported by the National Natural Science Foundation of China
Corresponding author: CHEN Chuan-sheng; Tel: +86-731-2309673; E-mail: jxccs1934@yahoo.com.cn