
Syntheses and cofluorescence of complexes of Eu(Ⅲ)/Y(Ⅲ) with terephthalic acid, 2-thenoyltrifluoroacetone and trioctylphosphine oxide
ZHAO Xue-hui(赵学辉)1, 2, HUANG Ke-long(黄可龙)1, LIU Su-qin(刘素琴)1,
JIAO Fei-peng(焦飞鹏)1, LIU Zhi-guo(刘志国)2, HU Shun-qin(胡舜钦)2, LI Zhao-jian(李朝建)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Green Packaging and Application Biological Nanotechnology of Hunan Province, Hunan University of Technology, Zhuzhou 412007, China
Received 25 May 2006; accepted 15 January 2007
Abstract: A series of Eu(Ⅲ)/Y(Ⅲ) complexes of terephthalic acid(TPA) with 2-thenoyltrifluoroacetone(HTTA) and trioctylphosphine oxide(TPPO) were synthesized. Compositions of these complexes are revealed to be Eu2(1-x)Y2x(TPA)(TTA)4- (TPPO)4 or Eu1-xYx(TPA)(TTA)(TPPO)2. Their IR spectra, fluorescent spectra and the thermal and optical stability were studied. The fluorescent excitation spectra of these complexes show more broad excitation bands than those of Eu2(TPA)3(TPPO)4 and Eu(TTA)3(TPPO)2 corresponding to their formations. In addition, both the binuclear complex EuY(TPA)(TTA)4(TPPO)4 and the chain complex Eu0.4Y0.6(TPA)(TTA)(TPPO)2 present higher thermal stability and better optical stability than the mononuclear complex Eu(TTA)3(TPPO)2 does. And their thermal and optical stabilities are preferably interpreted from the binuclear structure together with the chain polynuclear structure of the complexes. The fluorescence enhancement of cofluorescence Y3+ ions to the Eu(Ⅲ) complexes is clear. The optimum content of Y3+ is 0.6 (molar fraction) for the chain complexes Eu1-xYx(TPA)(TTA)- (TPPO)2 and 0.5 for the binuclear complexes Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4. The formation of polynuclear structure of the complex Eu1-xYx(TPA)(TTA)(TPPO)2 appears to be responsible for the good cofluorescence effect of Y3+ ions.
Key words: cofluorescence; europium; terephthalic acid; 2-thenoyltrifluoroacetone; trioctylphosphine oxide
1 Introduction
The organic complexes of rare earth ions having strong luminescent intensity, which have been found important application in light conversion events[1-2], are primarily attributed to both the unique electronic structure of rare earth ions and the antenna effect of organic ligands[3]. Generally, the fluorescence enhancement can be achieved through ligand sensitization. In this process, ultraviolet light is firstly absorbed by the organic ligands, then the absorbed energy may be transferred to the emitting rare earth ions and makes them emit their characteristic light. Organic ligands usually have a broad absorption band in the region of near ultra-violet. If the energy level of the triplet state of the ligand matches well with the emission energy level of rare earth ions, these will result in the great increase in luminescent intensity of rare earth ions[4-9]. In addition, the fluorescence enhancement can also be realized by the use of the photo-inertia lanthanide ions, such as Y3+, Gd3+ and La3+[10-13]. In the presence of these ions, the fluorescence enhancement of some lanthanide complexes can be obtained. This process is referred to cofluorescence, which was extensively studied in the mononuclear complexes of europiun(Ⅲ) ions with β-diketones ligands, such as Eu(TTA)3Phen[10] and Eu(TTA)3TPPO2[14].
Recently, the syntheses and luminescent properties of the mononuclear complexes of europium(Ⅲ) with 2-thenoyltrifluoroacetone(HTTA) and trioctylphosphine oxide(TPPO) were studied[1,14]. However, the studies on syntheses and luminescent properties of Eu(Ⅲ)/Y(Ⅲ) complexes of 2-thenoyltrifluoroacetone with terephthalic acid (TPA) and trioctylphosphine oxide have not been reported yet.
In this paper, the bridging ligand, terephthalic acid, is reported to be used to link Eu(Ⅲ)/Y(Ⅲ) ions to form the binuclear complexes Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4 and chain polynuclear complexes Eu1-xYx(TPA)(TTA)- (TPPO)2. The thermal and optical stability and fluorescent properties of above-mentioned complexes were studied, and the fluorescence enhancement of cofluorescence Y3+ ion for the Eu(Ⅲ) complexes was also investigated.
2 Experimental
2.1 Reagents and apparatus
99.99% Eu2O3 and 99.98% Y2O3 were purchased from Jiangxi South Rare Earth Metals Institute of China. 2-thenoyltrifluoroacetone(HTTA), terephthalic acid (TPA), trioctylphosphine oxide(TPPO) and other reagents were all analytical grade and used without further purification.
C, H analyses were performed on an American Perkin-Elmer 2400 II CHNSLO elemental analyzer. The contents of the rare earth were determined by complexometric titration with EDTA. The infrared spectra were measured at room temperature on Nicolet-550 spectrophotometer (American Perkin-Elmer) using KBr pellets in the spectral range of 4 000-400 cm-1. The scanning electronic microscopy(SEM) was obtained in a microscope JSM-5600LV along with the gold sputtering technique. Differential thermoanalysis (DTA) was performed in a SHDT-40 thermo- analyticmeter using aluminum crucibles with 18.40 mg of sample, under dynamic synthetic air atmosphere (40 mL/min) and heating rate of 10 ℃/min in the temperature range of 30-600 ℃. The thermogravimetric (TG) curves were recorded with a thermobalance model SHDT-40 in the temperature interval of 30-600 ℃, using platinum crucibles with 18.0 mg of sample, under dynamic synthetic air atmosphere (40 mL/min) and heating rate of 10 ℃/min. A Hitachi F-4500 Spectrometer was used to record excitation and emission spectra of the complex powders. The bandwidth of monochromators was set at 2.5 nm for both excitation and emission.
2.2 Synthesis of complexes
The complex Eu(TPA)(TTA)(TPPO)2 was prepared in the following steps. In the first step, standard solution of europium(Ⅲ) (1.0×10-1 mol/L) was prepared by dissolving Eu2O3 in hot hydrochloric acid, evaporating up to syrup and diluting with ethanol to a desired volume. HTTA, TPA and TPPO were dissolved separately in ethanol with molar ratio of 1?1?2. Subsequently the EuCl3 and HTTA solutions were mixed with molar ratio of 1?1, adjusting pH values to 5.5, stirred and refluxed for 0.6 h keeping temperature in water-bath. Then according to molar composition of formula Eu(TPA)(TTA)(TPPO)2, TPA and TPPO solutions were added dropwise, keeping pH value at 6.5, stirred and refluxed until appearance of a pale yellow precipitate. The solid product was filtered, washed with ethanol and distilled water successively, recrystallized in ethanol, and dried in a vacuum oven.
The Eu2(TPA)3(TPPO)4 and Eu2(TPA)(TTA)4- (TPPO)4 complexes were prepared by similar process as Eu(TPA)(TTA)(TPPO)2, except that the product for Eu2(TPA)(TTA)4(TPPO)4 was a yellow precipitate.
The complexes Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4 and Eu1-xYx(TPA)(TTA)(TPPO)2 were prepared by similar process as Eu(TPA)(TTA)(TPPO)2, except that the mixture of EuCl3 and YCl3 solution was used instead of EuCl3. The products obtained were a yellow or pale yellow powder.
The mononuclear complexes Eu(TTA)3(TPPO)2, Eu1-xYx(TTA)3(TPPO)2 were synthesized by similar process as described in Ref.[1]. The product obtained was a yellow powder.
3 Result and discussion
3.1 Composition of complexes
The rare earth contents were determined by complexometric titration with EDTA. Analytical data of C, H and rare earth contents for complexes are listed in Table 1. The elemental analytical data are consistent with the calculated values of the general formula of the Eu(Ⅲ) complexes.
Table 1 Elemental analysis data of complexes (mole fraction, %)

3.2 Characterization of complexes
Some results of IR spectra are shown in Table 2. The presence of carboxylate groups in the complexes was definitely confirmed by both the asymmetric stretching bands at 1 541-1 558 cm-1 and the symmetric stretching at 1395-1405 cm-1 The differences(?) between υas(COO) peaks and υs(COO) peaks are in the range of 142-156 cm-1 in the rare earth complexes, which are attributed to the chelating and bridging coordination modes of carboxylate groups with the rare earth since the difference (?=υas-υs) in the rare earth complexes is lower than that in Na2TPA (?=168 cm-1). In addition, owing to the great steric hindrance of the complexes Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4 and Eu1-xYx- (TPA)(TTA)(TPPO)2, the bridging coordination of carboxylate groups with the rare earth becomes more difficult than the chelating coordination. Thus, the coordination mode of carboxylate groups with rare earth ions is mainly the chelating coordination mode in these complexes, and the proposed chemical structures for the complexes Eu0.5Y0.5(TPA)(TTA)(TPPO)2 and EuY(TPA)- (TTA)4(TPPO)4 may be given in Fig.1. The IR spectra also show the displacement of υ(C=O) stretching from about 1 680 cm-1, in free TTA ligand, to approximately 1 608 cm-1 in the complexes, and the displacement of υ(P=O) stretching from 1 184 cm-1, in free TPPO ligand, to approximately 1 155 cm-1 in the complexes, indicating that Eu(Ⅲ)/Y(Ⅲ) ions are coordinated by the oxygen atoms[15].
Table 2 IR spectra data of some complexes


Fig.1 Chemical structures of complexes Eu0.5Y0.5(TPA)(TTA)- (TPPO)2 (a) and EuY(TPA)(TTA)4(TPPO)4 (b)
The complexes Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4
and Eu1-xYx(TPA)(TTA)(TPPO)2 present similar configuration, respectively. Fig.2 shows the agglomerated structure of the Eu2(TPA)(TTA)4(TPPO)4 complex and the chain/stripe structure of the Eu(TPA)(TTA)(TPPO)2 complex. For the binuclear complex Eu2(TPA)(TTA)4(TPPO)4, the formation of the agglomerated structure can be obtained by intermolecular force, while for the Eu(TPA)(TTA)- (TPPO)2 complex, the chain/stripe presents an infinite configurations, as suggested by the one-dimensional polymeric chain formed.

Fig.2 SEM images of Eu2(TPA)(TTA)4(TPPO)4 (a) and Eu(TPA)(TTA)(TPPO)2 (b) complexes
Fig.3 shows the DTA and TG curves in the temperature range from 50 to 600 ℃ for the Eu0.4Y0.6- (TPA)(TTA)(TPPO)2 and EuY(TPA)(TTA)4(TPPO)4. The DTA curve of the EuY(TPA)(TTA)4(TPPO)4 complex (Fig.3(a)) presents different profile to that of the Eu0.4Y0.6(TPA)(TTA)(TPPO)2 complex (Fig.3(b)). EuY(TPA)(TTA)4(TPPO)4 melts at about 213 ℃ and decomposes approximately in the temperature range from 290 to 385 ℃; Eu0.4Y0.6(TPA)(TTA)(TPPO)2 melts at about 235 ℃ and decomposes approximately in the temperature range from 357 to 412 ℃, both no decomposition before the melting point. The latter possess better thermal stability than the former. The DTA and TG curves do not present any event relative to water loss, in the interval 50-200 ℃, indicating that two complexes are in anhydrous form. This is corroborated by IR spectroscopy and elemental analysis. In addition, the temperature of the thermal decomposition of the mononuclear complex Eu(TTA)3(TPPO)2 is approximately in the interval 250 to 330 ℃, and the experiment result corroborates with that reported in Ref.[1]. So the thermal stability for the above mentioned Eu(Ⅲ) complexes increases in the following order: the mononuclear structure Eu(TTA)3(TPPO)2, the binuclear structure EuY(TPA)(TTA)4(TPPO)4, and the chain polynuclear structure Eu0.4Y0.6(TPA)(TTA)(TPPO)2.

Fig.3 DTA and TG curves of EuY(TPA)(TTA)4(TPPO)4 (a) and Eu0.4Y0.6(TPA)(TTA)(TPPO)2 (b) complexes
3.3 Fluorescent properties of complexes
The fluorescence excitation and emission spectra of the solid state complexes were performed on a Hitachi F-4500 Spectrometer.
The excitation spectra of the Eu(Ⅲ) complexes, recorded in the spectral range of 220-450 nm by monitoring the emission at the hypersensitive 5D0→7F2 transition, are shown in Fig.4. The excitation spectrum of Eu2(TPA)3(TPPO)4 consists of a broad band ranging from 240 to 340 nm with the maximum excitation wavelengths at 327 nm. The excitation spectrum of Eu(TTA)3(TPPO)2 shows a strong broad band ranging from 275 to 430 nm with the maximum excitation wavelengths at 383 nm. However, the data of the complexes Eu(TPA)(TTA)(TPPO)2 and Eu2(TPA)- (TTA)4(TPPO)4 exhibit a more broad band with two excitation peaks at about 346 nm and about 366 nm. In addition, in contrast to Eu(TTA)3(TPPO)2, the excitation band of TTA in Eu2(TPA)(TTA)4(TPPO)4 is shifted to shorter wavelength, and it is the same case for Eu(TPA)- (TTA)(TPPO)2. This indicates the TTA ligand has different coordination environments in the different complexes. Furthermore, compared with Eu2(TPA)- (TTA)4(TPPO)4, the excitation band of Eu(TPA)(TTA)- (TPPO)2 shows a peak position blue shift. These facts show the presence of the different orientation and different coordination environments for the TPA and TTA ligands in different states, corresponding to the formation of the Eu(Ⅲ)complexes.

Fig.4 Excitation spectra of complexes Eu2(TPA)3(TPPO)4 (a), Eu(TPA)(TTA)(TPPO)2 (b), Eu2(TPA)(TTA)4(TPPO)4 (c) and Eu(TTA)3(TPPO)2 (d)
The emission spectra were recorded in the range of 550-710 nm with the maximum excitation wavelengths at room temperature, as shown in Fig.5. It is noteworthy that similar emission spectra are observed for the Eu(Ⅲ) complexes. Five typical Eu(Ⅲ) emission bands appear approximately at about 583, 594, 616, 653, 703 nm, corresponding to 5D0→7F0, 5D0→7F1, 5D0→7F2, 5D0→7F3, and 5D0→7F4, respectively. Of all the fluorescent emissions for the Eu(Ⅲ) complexes, the relative fluorescence intensity of 5D0→7F2 is the strongest.

Fig.5 Emission spectra of complexes: (a) Eu2(TPA)3(TPPO)4; (b) Eu(TPA)(TTA)(TPPO)2; (c) Eu2(TPA)(TTA)4(TPPO)4; (d) Eu(TTA)3(TPPO)2
The data in Table 3 show the relative fluorescence intensity for the interesting Eu(Ⅲ) complexes studied. And the fluorescence intensity (5D0→7F2) for the interesting Eu(Ⅲ) complexes increases in the following order: Eu2(TPA)3(TPPO)4, Eu(TPA)(TTA)(TPPO)2, Eu0.4Y0.6(TPA)(TTA)(TPPO)2, Eu2(TPA)(TTA)4(TPPO)4, Eu(TTA)3(TPPO)2, and EuY(TPA)(TTA)4(TPPO)4. The results demonstrate that the fluorescence intensity of the Eu(Ⅲ) complexes is influenced by the structure of complex together with the species and coordination environments of ligand.
Table 3 Data of fluorescence spectra and ratios of relative intensity 5D0→7F2 and 5D0→7F1 for complexes

The ratios of the relative intensity 5D0→7F2 and 5D0→7F1 are also shown in Table 3. By comparison, it can be seen that the symmetry of the new complexes changes much less, i.e. the forced electric dipole transition (5D0→7F2) of Eu3+ in the complexes is strengthened, and the color purity of the assembly luminescence is significantly improved.
3.4 Effect of cofluorescence Y3+ ions on fluorescence intensity
The effects of Y3+, La3+ and Gd3+ on the fluorescence intensity of the complexes are similar, only the result of Y3+ is shown in Fig.6. It can be seen from Fig.6, firstly, that the fluorescence intensity of the complexes Eu1-xYx(TPA)(TTA)(TPPO)2, Eu2(1-x)Y2x- (TPA)(TTA)4(TPPO)4 and Eu1-xYx(TTA)3(TPPO)2 increases with the increment of Y3+ content (molar fraction). However, when Y3+ content is above 0.6, the fluorescence intensities of the complexes decrease with the increment of Y3+ content. But the ability of Y3+ for fluorescence enhancement of the Eu(Ⅲ) complexes is clear. The optimum content of Y3+ is 0.6 (molar fraction) for the chain complexes Eu1-xYx(TPA)(TTA)- (TPPO)2, 0.5 for the binuclear complexes Eu2(1-x)Y2x(TPA)(TTA)4- (TPPO)4 and 0.4 for the mononuclear complexes Eu1-xYx(TTA)3(TPPO)2. The effect of Y3+ on fluorescent enhancement of the above mentioned Eu(Ⅲ) complexes increases in the following order: Eu1-xYx(TTA)3(TPPO)2, Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4, and Eu1-xYx(TPA)- (TTA)(TPPO)2. The ability of Y3+ for fluorescence enhancement of the above mentioned Eu(Ⅲ) complexes may be concerned with the mononuclear, binuclear and chain structure of the complexes.
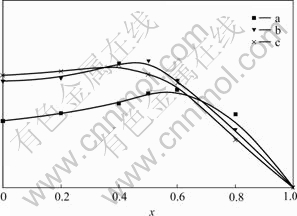
Fig.6 Effect of Y3+ content on fluorescence intensity of complexes: (a) Eu1-xYx(TPA)(TTA)(TPPO)2; (b) Eu2(1-x)- Y2x(TPA)(TTA)4(TPPO)4; (c) Eu1-xYx(TTA)3(TPPO)2
3.5 Optical stability of complexes
Some optical stability tests were also carried out using 360 nm ultraviolet light for complexes EuY(TPA)(TTA)4(TPPO)4, Eu0.4Y0.6(TPA)(TTA)(TPPO)2 and Eu(TTA)3(TPPO)2.
It can be seen in Fig.7 that the optical stability of the Eu(Ⅲ) complexes increases in this order: mononuclear structure Eu(TTA)3(TPPO)2, binuclear structure EuY(TPA)(TTA)4(TPPO)4, and chain structure Eu0.4Y0.6(TPA)(TTA)(TPPO)2.

Fig.7 Changes of fluorescence intensity with irradiation time for complexes: (a) Eu(TTA)3(TPPO)2; (b) EuY(TPA)(TTA)4- (TPPO)4; (c) Eu0.4Y0.6(TPA)(TTA)(TPPO)2
4 Conclusions
1) A series of relative cheap light conversion complexes of Eu(Ⅲ)/Y(Ⅲ) with 2-thenoltrifluoro- acetone, terephthalic acid and trioctylphosphine oxide, showing the good thermal and optical stability together with strong red fluorescence, were synthesized. Compositions of these complexes are revealed to be Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4 or Eu1-xYx(TPA)(TTA)- (TPPO)2 (x=0-1).
2) The thermal and optical stability for the Eu(III) complexes increases in the following order: mononuclear complex Eu(TTA)3(TPPO)2, binuclear complex EuY(TPA)(TTA)4(TPPO)4, and chain complex Eu0.4Y0.6- (TPA)(TTA)(TPPO)2. The formation of the binuclear/ chain polynuclear structure of the complexes appears to be responsible for the enhancement of the thermal and optical stability of the complexes.
3) The fluorescence enhancement of the Eu(Ⅲ) complexes can be obtained by the addition of relative cheap Y3+ ions. The optimum content of Y3+ is 0.6 (molar fraction) for the chain polynuclear complexes Eu1-xYx(TPA)(TTA)(TPPO)2, 0.5 for the binuclear complexes Eu2(1-x)Y2x(TPA)(TTA)4(TPPO)4 and 0.4 for the mononuclear complexes Eu1-xYx(TTA)3(TPPO)2.
References
[1] FU Y J, WONG T K S, YAN Y K, HU X. Syntheses, structures and luminescent properties of Sm(Ⅲ) and Eu(Ⅲ) chelates for organic electroluminescent device applications [J]. J Alloys Compounds, 2003, 358: 235-244.
[2] LIU H G, SEONGTAE P, KIWAN J, FENG X S, CHANGDAE K, SEO H J, LEE Y I. Influence of ligands on the photoluminescent properties of Eu3+ in europium β–diketonate/PMMA-doped systems [J]. Journal of Luminescence, 2004, 106: 47-55.
[3] ANDRZEJ M K, STEFAN L, ZBINGNIEW H, KATARZYNA C, MAREK P, MARIAN E. Improvement of emission intensity in luminescent materials based on the antenna effect [J]. J Alloys Compounds, 2000, 300/301: 55-60.
[4] BRITO H F, MALTA O L, MENEZES J F S. Luminescent properties of diketonates of trivalent europium with dimethylsulfoxide [J]. J Alloys Compounds, 2000, 303/304: 336-339.
[5] YANG J H, ZHU G Y, WU B. Enhanced luminescence of the europium/terbium/thenoyltrifluoroacetone/1,10-phenanthroline/surfactant system and its analytical application [J]. Anal Chim Acta, 1987, 198: 287-291.
[6] YANG J H, REN X Z, ZHOU H B, SHI R P. Enhanced luminescence of the europium(Ⅲ)—dibenzoylmethane-ammonia-acetone system and its analytical application to the determination of europium ion [J]. Analyst, 1990, 115: 1505-1509.
[7] PANIGRAHI B S. Fluorescence and cofluorescence enhancement of Tb3+ and Eu3+ using phenyl phosphonic and phenyl phosphinic acids as ligands [J]. Journal of Luminescence, 1999, 82: 121-127.
[8] PANIGRAHI B S. A fluorimetric study of terbium, europium and dysprosium in aqueous solution using pyridine carboxylic acids as ligands [J]. J Alloys Compounds, 2002, 334: 228-231.
[9] PANIGRAHI B S, SUSY P, VISWANATHAN K S, MATHEWS C K. Fluorescence enhancement of Tb3+ in Tb-aromatic acid complexes: Correlation of synergistic enhancement with the structure of the ligand [J]. Spectrochimica Acta (Part A), 1995, 51: 2289-2300.
[10] WANG Zheng-xiang, SHU Wan-yin, ZHOU Zhong-cheng, LIU You-nian, CHEN Hong. Fluorescence properties and application of doping complexes Eu1-xLx(TTA)3Phen as light conversion agents [J]. J Cent South Univ Technol, 2003, 10(4): 342-346.
[11] JIU H F, DING J J, BAO J, ZHANG Q, CAO C. Combinatorial method for the study of new cofluorescence enhancement system [J]. Spectrochimica Acta (Part A), 2005, 61: 3150-3154.
[12] PANIGRAHI B S, SUSY P, VISWANATHAN K S. Cofluorescence of Eu3+ in complexes of aromatic carboxylic acids [J]. Spectrochimica Acta (Part A), 1997, 53: 2579-2585.
[13] YANG J H, ZHOU H B, REN X Z, LI C Y. Fluorescence enhancement of the Eu-Tb-Benzoylacetone- phenanthroline system [J]. Anal Chim Acta, 1990, 238: 307-402.
[14] FU Y J, WONG T K S, YAN Y K, WANG G M, HU X. Syntheses, characterization and luminescent properties of Eu(Ⅲ) complex [J]. Thin Solid Films, 2002, 417: 78-84.
[15] MARIA CLAUDIA F C F, CLAUDIA S T, HERMI F B, ERCULES E S T, OSCAR L M. Synthesis and luminescent properties of supramolecules of β-diketonate of Eu(Ⅲ) and crown ethers as ligands [J]. J Solid State Chemistry, 2003, 171: 189-194.
Foundation item: Project(20576142) supported by the National Natural Science Foundation of China; Project(05B075) supported by the Foundation of Hunan Education Bureau for Young Scholars, China
Corresponding author: HUANG Ke-long; Tel: +86-731-8832457; E-mail: klhuang@mail.csu,edu.cn
(Edited by YUAN Sai-qian)