
Formation of interfacial brittle phases sigma phase and IMC in hybrid titanium-to-stainless steel joint
Min Ku LEE, Jung Gu LEE, Jong Keuk LEE, Sung Mo HONG, Sang Hoon LEE,
Jin Ju PARK, Jae Woo KIM, Chang Kyu RHEE
Nuclear Materials Research Division, Korea Atomic Energy Research Institute, Daejeon 305-353, Korea
Received 21 April 2010; accepted 10 September 2010
Abstract: The microstructures of the brazed joints for commercially pure Ti and stainless steel were investigated by the applications of various filler alloys including Ag-, Ti-, Zr- and Ni-based alloys. Generally, the dissimilar joints between Ti and stainless steel were dominated by the Ti-based intermetallic compounds (IMCs), e.g. (Ti, Zr)2(Fe, Ni), TiFe, TiCu, and Ti2(Fe, Ni), due to a significant dissolution of Ti from the base metal. The (Fe-Cr) s phase was also observed near the stainless steel due to a segregation of Cr into the interface region. This research demonstrates empirically that the brittleness of the Ti and stainless steel joint can not be avoided only by applying single braze alloy or single insert metal, and thus an introduction of additional suitable interlayer between the filler alloy and the base metal is necessary to prevent the brittleness of the joint.
Key words: brazing; titanium; stainless steel; intermetallic compounds (IMCs)
1 Introduction
The utilization of costly metals and alloys with excellent properties is increasing. As a result, there is a demand for methods to join these expensive metals with the more commonly used metals. This requirement is not always caused by the cost, but also by the desire for new, basically improved structures. Joining dissimilar metals also enables us to use the most suited materials for each part of the structure. Titanium and its alloys have been considered one of the best engineering materials for their use in various industrial applications[1-2]. A main cause is their excellent corrosion resistance, which is capable of being comparable to platinum, due to a stable, protective and strongly adherent oxide film layer[3]. With the increasing use of Ti, the joining technology of Ti and its alloys has become more important, particularly their joining to various structural iron-base steel alloys.
The present study reports the typical brazing characteristics and origin of the brittleness for the commercially pure Ti-to-stainless steel (STS) joints by employing various filler alloy classes including Ag-based, Ti-based, Zr-based and Ni-based alloys. Discussions are also made in view of an introduction of the interlayer metal as an interfacial diffusion control layer for achieving strong Ti-to-STS brazed joints.
2 Experimental
The base materials used for a dissimilar joining were commercially pure Ti (Gr. 2) and super stainless steel (UNS S31254), and were rectangular cubes with dimensions of 10 mm ? 10 mm ? 15 mm. UNS S31254 is one of the super stainless steels containing 20Cr-18Ni-6Mo (mass fraction, %).
The infrared brazing was employed as a joining technique, because it is characterized by an extraordinarily fast heating rate up to 3 000 °C/min, and thus the interfacial reaction between the molten filler and the solid base metals may be considerably decreased due to a rapid thermal history[4-5]. The chamber was evacuated up to approximately 6.7 mPa and then purged with Ar gas at a flow rate of 4 L/min. After this, the infrared heating was started with a heating rate of 100 °C/min, and the specimen was isothermally held at a fixed temperature, finally followed by a cooling at 100 °C/min. During the joining, the specimens were compressively loaded with 0.13 MPa.
The microstructure and quantitative chemical analyses for the cross-sections of the joints were performed by SEM (JEOL 6300) equipped with an energy dispersive spectroscopy (EDS) with an operating voltage of 20 kV and a spot size of 1 mm. The room temperature tests were performed to evaluate the bonding strength with a tensile testing machine (INSTRON MODEL 4465) at a strain rate of 8.3?10-4 s-1.
3 Results and Discussion
Generally, brazing cycles of titanium and its alloys are limited by their bulk property, which is “beta transus”, i.e. the critical temperature for a-to-b phase transformation. As their structure and properties are impaired above the beta transus, the filler metals with brazing temperatures below the beta transus are preferable. All known titanium brazing filler metals can be grouped by five major families, as shown in Fig.1, and they are aluminum-, silver-, zirconium-, titanium-, and palladium-based alloys. The aluminum-based filler and some of the silver-, zirconium-, and titanium-based alloys may satisfy the criteria that the brazing is preferred at temperature lower than the beta transus. As the brazed joints by the Al-based filler are known to be very brittle with low fatigue resistance and impact strength[6], temperature much more than 1 100 °C, those two groups of filler alloys are ruled out in this study. Besides three groups of filler alloys (silver-, zirconium-, titanium-), the Ni-based alloys are also introduced for dissimilar brazing Ti and STS, despite their high melting temperatures exceeding 1 000 °C, since they are currently untilized in brazing STS . The composition and melting temperature properties for the four groups of filler alloys used in this study and the corresponding infrared brazing conditions are summarized in Table 1.
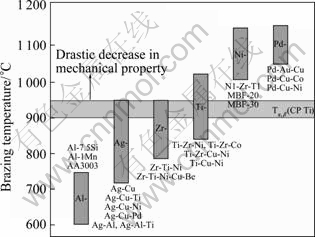
Fig.1 Basic classes of filler alloys for brazing titanium and its alloys[6]
The SEM back-scattering electron images (BEIs) for the Ti-STS dissimilar joints with regard to Ag-, Ti-, Zr-, and Ni-based filler alloys are shown in Figs.2(a)-(h), respectively. The resultant interfacial brittle phases and the joint strength brazed by various filler alloys are summarized in Table 2. From the experimental observation, the dissolution of the Ti substrate was much more prominent than that of the STS substrate, thus the joint was mainly involved in an intensive reaction of the dissolved Ti with the molten filler elements. For the Ag-based filler alloys, the joint was mainly dominated by thick Ti2Cu, TiCu and Ti3Cu4 IMC layers, along with a formation of the segregated Ag-rich phase region in the center of the joint. The TiFe and TiCu IMCs were also produced at the STS interface (Figs.2(a) and (b)). This is a typical microstructure formed by using Ag-based filler alloys. The tensile strength of the joint sample was as low as about 60 MPa and the fracture occurred along the brittle IMCs.
In case of the Ti- and Zr-based filler alloys, the similar microstructures are observed, as shown in Figs.2(b)-(f). After melting of the filler, the isothermal solidification started near the Ti interface due to dissolution of Ti from the base metal and the induced change of composition. So, the b-Ti and (a+b)-Ti region continued to form and the remnant elements were segregated close to the STS interface. The segregated layer was found to be the brittle IMC, i.e. (Ti, Zr)2(Fe, Ni). Finally, the resultant joint was typically composed of (Ti, Zr)2(Fe, Ni) layer, b-Ti region, and (a+b)-Ti from STS towards Ti. When the brazing temperature became higher, the Fe-Cr s phase was also observed in the vicinity of the STS interface, probably owing to the segregation of Cr in the base metal towards the interface. The tensile strength of the joint sample was in the range of 50-280 MPa. The fracture occurred at (Ti, Zr)2(Fe, Ni) layer or the interface between the (Ti, Zr)2(Fe, Ni) and Fe-Cr s phases.
Table 1 Composition of filler alloys used and infrared brazing conditions
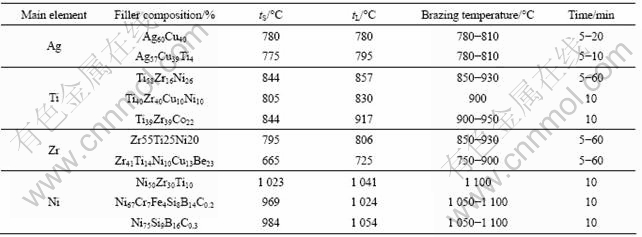
Table 2 Summary on resultant interfacial brittle phase and joining strengths measured by room temperature tensile tests for samples brazed by various filler alloys

As shown in Figs.2(g) and (h), the joint regions were very thick with about 400 mm, when the Ni-based alloys were applied. This is because the dissolution of base applied. This is because the dissolution of base metals was significant due to higher brazing temperature more than 1 000 °C. The joint had typical brittle phases of (FeTi, NiTi) and Ti2(Fe, Ni), and was composed of a region of b-Ti + (FeTi, NiTi) + Ti2(Fe, Ni), a region of b-Ti + Ti2(Fe, Ni), and Ti-rich solid solution. The tensile strength of the sample was less than 70 MPa.
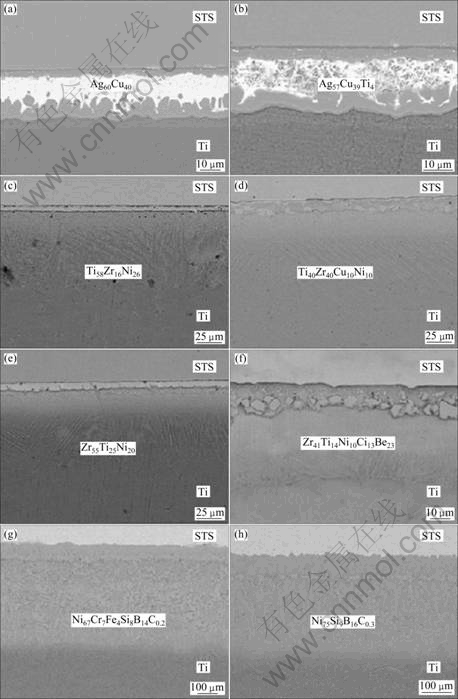
Fig.2 SEM images showing typical microstructure with respect to various filler alloys: (a), (b) Ag-based; (c), (d) Ti-based; (e), (f) Zr-based; (g), (h) Ni-based alloys
From the above investigations, it is suggested that the Ti-to-STS joints were very brittle with poor bonding strength regardless of the filler due to the formations of the stable brittle IMCs and s phases, and their fundamental brittleness might be inevitable only by applying single braze alloy or single insert metal. This is also understood well from the knowledge of the mutual solubility between the two elements of the periodic system, as shown in Fig.3. The Ti readily reacts with most other elements to form the stable and brittle IMC phases, except for a few elements in the same or adjacent groups of the periodic system (V, Zr, Nb, Ta, etc). Especially, it forms the most brittle Ti-Fe IMCs among Ti-based IMCs with the main element Fe in STS. Unfortunately, none of the elements is highly soluble at the same time in both the Ti and Fe over the whole period system.
The above investigations lead to a consideration that another interlayered structure between the filler alloy and the base metal may be necessary to prevent such a fundamental brittleness. One of the candidates for suitable interlayer metals may be vanadium (V). The b-Ti forms a complete range of solid solutions with the elements V, Nb, and Ta, whereas the behavior of a-Ti is more limited in this respect. At 700 °C, 3% V (in mass fraction) can be dissolved in a-Ti. Vanadium dissolves 87% Ti at this temperature[7]. These promising properties are further enhanced by thermal expansion coefficients with a molar ratio (Ti : V) of 8.5 : 8.3. Thus, V can be used as a suitable insert or interlayer metal.
In order to utilize the V interlayer for Ti-to-STS joint, the compatibility of V with the STS base metals should also be considered. Vanadium forms only with alpha Fe a complete series of solid solutions. In γ-Fe, its solubility is rather limited and IMC phases are present. Only Cr has an unlimited solubility in V, whereas Ni has only a limited solubility to form V-Ni IMC phases. Another important consideration can be made on a formation of the stable V-Fe s phase in the composition range from 30% V to 60% V(molar fraction) at temperatures below 1200 °C[7-8]. Since the s phase exhibits generally deleterious effects on the mechanical behavior due to its brittle nature, it should be also eliminated from the joint. For this, the Cr and Ni metals may be applied consecutively as interlayers between the V layer and STS. The Cr is highly soluble with Ni[7], and at the same time exhibits a complete range of solid solutions with V[7]. The Ni is also applied between Cr interlayer and STS to suppress the formation of the Fe-Cr s phase. It is, therefore, suggested that three layers of Ni-Cr-V may be suitable as interlayers for achieving a strong Ti-to-STS joint without any brittle IMC or s phase.
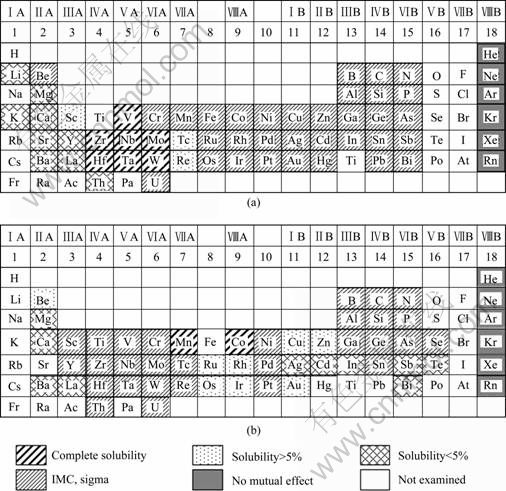
Fig.3 Mutual solubility properties of Ti (a) and Fe (b) with other elements in binary alloy periodic system (up to 1 200 °C)
4 Conclusions
1) The dissimilar brazing characteristic between the commercially pure Ti and stainless steel (STS) was investigated, with regard to various filler alloys including Ag-based, Ti-based, Zr-based and Ni-based alloys.
2) The joint was mainly involved in an intensive reaction of the dissolved Ti with the molten filler elements, and the Ti-based brittle IMC phases formed.
3) The vanadium is proposed as a suitable interlayer metal to prevent the brittle IMC in the Ti-to-STS dissimilar joints, and the Ni and Cr are recommended as interlayers between the V and STS to avoid the brittle V-Fe and Fe-Cr sigma phases.
Acknowledgements
This research was financially supported by the
Korea Atomic Energy Research Institute (KAERI) R&D Program.
References
[1] KAHRAMAN N, GULENC B, FINDIK F. Corrosion and mechanical-microstructural aspects of dissimilar joints of Ti-6Al-4V and Al plates [J]. International Journal of Impact Engineering, 2007, 34: 1423-1432.
[2] BARRENDA J L, SANTAMARIA F, AZPIROZ X, IRRISARRI A M, VARONA J M. Electron beam welded high thickness Ti6Al4V plates using filler metal of similar and different composition to the base plate [J]. Vacuum, 2001, 62: 143-150.
[3] LEE H S, YOON J H, YI Y M. Oxidation behavior of titanium alloy under diffusion bonding [J]. Thermochimica Acta, 2007, 455: 105-108.
[4] SHIUE R K, WU S K, CHEN S Y. Infrared brazing of TiAl intermetallic using BAg-8 braze alloy [J]. Acta Materialia, 2003, 51: 1991-2004.
[5] SHIUE R K, WU S K, CHEN S Y. Infrared brazing of TiAl using Al-based braze alloys [J]. Intermetallics, 2003, 11: 661-671.
[6] SHAPIRO A, RABINKIN A. Stste of the art of titanium-based brazing filler metals [J]. Welding Journal, 2003, 83(10): 36-43.
[7] MASSALSKI T B. Binary alloy phase diagram [M]. USA: Metals Park, ASM International, 1990: 3495, 1790, 1301, 1352.
[8] SEKI J, HAGIWARA M, SUZUKI T. Metastable order-disorder transition and sigma phase formation in Fe-V binary alloys [J]. Journal of Materials Science, 1979, 14: 2404-2410.
(Edited by HE Xue-feng)
Corresponding author: Min Ku LEE; Fax: +82-42-868-4847; E-mail: leeminku@kaeri.re.kr