J. Cent. South Univ. Technol. (2008) 15(s1): 158-162
DOI: 10.1007/s11771-008-337-8
Effect of surface treatment on rheological behaviors of glass bead/hydroxyl terminated polybutadiene suspensions
CUI Wei(崔 伟)1, 2, ZHAO Jin-chao(赵瑾朝)1, 2, WANG Cheng-zhe(王承哲)1, 2,
ZHOU Xing-ping(周兴平)1, 2, XIE Xiao-lin(解孝林)1, 2
(1. School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology,
Wuhan 430074, China;
2. Hubei Key Laboratory of Materials Chemistry and Service Failure, Huazhong University of Science and Technology,
Wuhan 430074, China)
Abstract: Surface treatment of glass bead (GB) was carried out by using γ-glycidoxypropyltrimethoxy silane (GPTES) and γ-methacryloxypropyltrimethoxy silane (MPTMS) as coupling agents, respectively. The steady viscosity and yield stress of the GB/hydroxyl terminated polybutadiene (HTPB) suspensions were determined by Brookfield R/S rheometer. The effect of surface treatment on the viscosity and yield stress of GB/HTPB suspension was investigated. The results indicate that the viscosity of the pristine GB/HTPB suspension increases with increasing GB, and the relationship between its viscosity and volume fraction of GB depends on the shear rate. The modification of GB by MPTMS changes the viscosity of the MPTMS@GB/HTPB suspension, and its viscosity is the minimum at the MPTMS dosage of 0.3?g per 1 g GB. Additionally, the modification of GB by MPTMS increases the yield stress of the GB/HTPB suspension, and its yield stress is the maximum at the MPTMS dosage of 0.1?g per 1 g GB. The GPTES modified GB/HTPB suspension behaves lower viscosity and weaker shear thinning than the MPTMS modified GB/HTPB suspension within the range of experimental shear rate.
Key words: glass bead; suspensions; coupling agent; steady shear viscosity; yield stress
1 Introduction
Concentrated suspension is a non-Newtonian fluid, its rheological properties play a very important role on the preparation and processing of solid propellants, ceramics, coatings, inks and composites. Based on the effects of the loading fraction, the size and size distribution of inorganic particle, and the interfacial adhesion between inorganic particles and polymer matrix on the rheological properties of the suspension[1-4], scientists proposed a series of constitutive equations to describe its viscosity and yield stress[5-9]. For example, Krieger—Dougherty relation[8] and Maron—Pierce equation[9] are widely used to calculate the maximum packaging volume fraction (φmax)[10] and the thickness of the polymer layer absorbed on particle surface[11], and describe the polymer-inorganic particle affinity[12].
Coupling agents (CA) are used to modify the surface of inorganic particles in order to improve their dispersion and the interfacial adhesion between particles and the polymer matrix[13]. The CA molecules covered on particle surface via physically adsorption or chemically bonding decrease the interaction between particles, which results in that the particles exhibit the characters of hard-spheres[14], the effective volume fraction (φeff) and rheological behavior of the suspension are greatly changed. Unfortunately, little information is available on the relationship between CA treatment and rheological behaviors of the concentrated suspension. In the present work, the glass bead (GB) was modified by different coupling agents, such as γ-glycidoxypropyltrimethoxy silane (GPTES) and γ-methacryloxypropyltrimethoxy silane (MPTMS), respectively. The effect of surface treatment on the viscosity and yield stress of GB/ hydroxyl terminated polybutadiene (HTPB) suspension was investigated.
2 Experimental
2.1 Materials
Glass bead (GB) powders, with average particle size of 2.14 μm, specific area of 1.47?m2/g and density of 2.49?g/cm3, were provided by Beijing Anjilian Technol. Co. Ltd., China. Hydroxyl terminated polybutadiene (HTPB), with average relative molar mass of 4 120?g/mol and density of 0.9?g/cm3, was provided by Shanghai Reagents Company, China. Two coupling agents, γ-glycidoxypropyltrimethoxy silane (GPTES) and γ-methacryloxypropyltrimethoxy silane (MPTMS), were commercially available from Wuhan University Silicon New Material Co., Ltd., China.
2.2 Surface modification of GB
According to Ref.[15], the GB surface was modified by GPTES and different dosage of MPTMS, respectively. The received modified GB powders were denoted as GPTES@GB and MPTMS@GB.
2.3 Preparation of GB/HTPB Suspensions
The unmodified GB and modified GB were respectively compounded with HTPB in ice bath under high blending speed of 2 000?r/min for 1 h. Then, the suspensions were kept in a vacuum oven at 50?℃ for 4?h to remove the trapped air bubbles. The mass fraction of GB varied from 30%-70%.
2.4 Characterization
Fourier transform infrared spectroscopy (FT-IR, Equinox?55, Bruker, Germany) was applied to characterizing the chemical structure of GB after surface modification. Thin film specimens were pressed with KBr powders.
In order to characterize the hydrophobicity of the modified GB surface, the glass flake (GF) was used to mimic GB powders according to the method in Ref.[12]. According to Ref.[15], the GF surface was modified as the same as GB powders. The received modified GFs were denoted as GPTES@GF and MPTMS@GF. Then, the contact angle meter (CA-XP150, Consonance Interface, Japan) was applied to determining the contact angle of water on the above flakes.
The rheological behaviors of GB/HTPB suspensions were measured by a Brookfield R/S rheometer (Brookfield, USA). For steady shear experiment, a coaxial cylinder geometry (CC-14) at controlled shear rate mode was used to test the viscosity of suspensions. Their yield stress values were measured by a vane geometry (V40-20) under a constant shear rate of 0.1?s-1 according to Ref.[16].
3 Results and discussion
3.1 Surface characteristic of GB
Fig.1 shows the FT-IR spectra of pristine GB, MPTMS@GB and GPTES@GB (the dosages of MPTMS and GPTES were 0.03?g per 1 g GB). In curve 2, the peak at 1 720?cm-1 attributes to the absorption band of carbonyl group in MPTMS, and vibration peaks at 2 962, 2 929 and 2 858?cm-1 are related to the absorption bands of methyl and methylene groups in MPTMS. Similarly, in curve 3, the strong peak at 907?cm-1 corresponds to the absorption band of the epoxy group in GPTES, and the peaks at 2 939?and 2 880?cm-1 attribute to the vibration absorption bands of methyl and methylene groups in GPTES. The above characteristic absorption peaks indicate that MPTMS and GPTES molecules are covered on GB surface via Si— O—Si bonds.
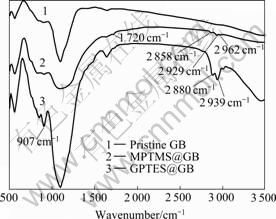
Fig.1 FT-IR spectra of three samples
Table 1 lists the contact angles of water on pristine and modified GFs. Apparently, the contact angles of MPTMS@GF and GPTES@GF are larger than that of pristine GF, which indicates that the surface modification increases the hydrophobicity of GFs and beads.
Table 1 Contact angle data of pristine and modified GFs

3.2 Dependence of relative viscosity of GB/HTPB suspension on volume fraction of GB
Fig.2 shows the relationship between the relative viscosity of pristine GB/HTPB suspension and volume fraction of GB under shear rate of 2?s-1 at 20?℃.
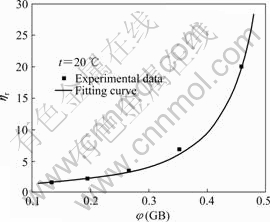
Fig.2 Relative viscosity of pristine GB/HTPB suspensions as function of volume fraction of GB under shear rate of 2?s-1
It can be seen from Fig.2 that the relative viscosity of the suspension increases with increasing loading fraction of GB, and it increases more obviously at high loading fraction of GB. Generally, the addition of inorganic particles into polymer matrix will restrict the movement of polymer chain segments, leading to an increase in flow resistance and the viscosity of the suspension. By fitting the relation between the relative viscosity and volume fraction of pristine GB/HTPB suspension with Maron—Pierce equation, the maximum volume fraction (φmax) is calculated to be 0.591. Thereby, the Maron—Pierce equation for the pristine GB/HTPB suspension is described as
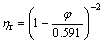
For the suspension with monodisperse hard spheres, the φmax equals 0.68 theoretically[6], and higher than φmax of the pristine GB/HTPB suspension, which indicates that there exists strong aggregation of GB particles in suspension.
The relative viscosity of pristine GB/HTPB suspension as function of volume fraction of GB at various shear rates is shown in Fig.3.
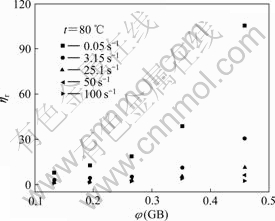
Fig.3 Relative viscosity of pristine GB/HTPB suspensions as function of volume fraction of GB at various shear rates
Apparently, the relative viscosity of the pristine GB/HTPB suspension increases sharply with the volume fraction of GB under low shear rate. Since the ηr—φ relationship is mainly controlled by the combined effects of the inter-particulate interaction and hydrodynamic force for the suspension containing the inorganic particle with the diameter larger than 1?μm[17], the inter- particulate interaction is predominant at low shear rate in suspension, and the weak hydrodynamic force can not efficiently break the aggregation of inorganic particles especially at high loading, thus resulting in a sharp increase in viscosity of the pristine GB/HTPB suspension. However, with increasing shear rate, the hydrodynamic force plays a predominant role on the suspension, thus resulting in a decrease in viscosity of the pristine GB/HTPB suspension, and ηr almost does not vary with φ under high shear rate.
3.3 Effect of surface treatment on viscosity of GB/HTPB suspension
Fig.4 represents the viscosity curves of MPTMS@ GB/HTPB suspensions with 30% GB modified by different dosages of MPTMS at 20?℃. It can be seen that the MPTMS@GB/HTPB suspensions exhibit shear- thinning behavior within the range of experimental shear rate, and their viscosity is lower than that of the pristine GB/HTPB suspension except the suspension with MPTMS dosage of 0.1?g per 1g GB.
Fig.5 shows the variation of viscosity of the MPTMS@GB/HTPB suspensions containing 30% GB with different dosages of MPTMS under 39?s-1 at 20?℃.
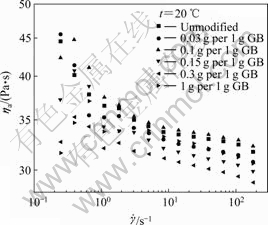
Fig.4 Viscosity curves of GB/HTPB suspensions with 30% GB modified by different dosage of MPTMS
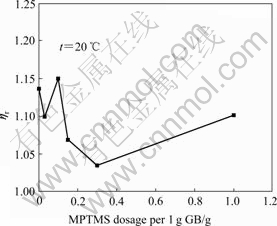
Fig.5 Variation of viscosity of MPTMS@GB/HTPB suspension containing 30% GB with different dosages of MPTMS under shear rate of 39?s-1
Initially, its viscosity decreases after GB particles are modified by MPTMS of 0.03?g per 1 g GB. When the MPTMS dosage increases to 0.1?g per 1 g GB, the viscosity of the MPTMS@ GB/HTPB suspension increases to a maximum. However, its viscosity decreases with further increasing MPTMS dosage. When the MPTMS dosage is 0.3?g per 1 g GB, the viscosity of the MPTMS@ GB/HTPB suspension is minimum. Later, its viscosity increases again with increasing the MPTMS dosage. The phenomenon was also observed in poly(ethylene oxide) modified colloidal silica/water suspensions[18].
As discussed above, the modification by MPTMS increases the hydrophobicity of GB particles, enhances the interfacial interaction between GB and HTPB, breaks that the aggregation of GB particles and improves the dispersion of GB particles in HTPB. The above factors lead to the decrease in the viscosity of MPTMS@GB/ HTPB suspension. However, when the MPTMS dosage is 0.1?g per 1 g GB, the increase in the flow units due to the improved dispersion of GB particles increases the viscosity of the MPTMS@GB/HTPB suspension to a maximum. With further increasing the MPTMS dosage, the size and number of GB flow units will no longer change due to the balance between the inter-particulate interaction and hydrodynamic force in suspension. On the other hand, MPTMS molecules covered on the GB surface enhance the wetting of HTPB on GB surface. When the MPTMS dosage is 0.3?g per 1 g GB, these MPTMS molecules form a monolayer oriented along the direction parallel to the GB surface (see the top structure in Fig.6), and decrease the inter-particulate interaction to minimum. Thereby, the modified GB particles behave like the “smoother” hard spheres, resulting in a sharp decrease in viscosity of the MPTMS@GB/HTPB suspension. The MPTMS dosage of 0.3?g per 1 g GB is very close to the dosage 0.4?g per 1 g silica of MPTMS molecules that form a monolayer oriented along the direction parallel to the silica surface[19]. When the MPTMS dosage is more than 0.3?g per 1 g GB, these MPTMS molecules form a coverage layer oriented along the direction perpendicular to the GB surface (see the bottom structure in Fig.6), and increase the hydrodynamic volume of GB particle and the viscosity of the MPTMS@GB/HTPB suspension. BAUER et al[20] modified the nano-silica particles by MPTMS of 0.5?g per 1 g silica, and confirmed the perpendicular orientation structure of MPTMS layer on nano-silica surface by 29Si CP-MAS NMR.
3.4 Effect of surface treatment on yield stress of GB/HTPB suspension
The variation of yield stress of the MPTMS@ GB/HTPB suspension containing 30% GB with different MPTMS dosages at 20?℃ is shown in Fig.7. It can be seen that the yield stress of the MPTMS@GB/HTPB suspension is higher than that of the pristine GB/HTPB suspension. With increasing MPTMS dosage, the yield stress of the MPTMS@GB/HTPB suspension initially increases. When the MPTMS dosage increases to 0.1?g per 1 g GB, its yield stress increases to a maximum. However, with further increasing the MPTMS dosage, the yield stress of the MPTMS@GB/HTPB suspension decreases. As discussed above, the modification by MPTMS improves the dispersion of GB particles in HTPB, and enhances the interfacial interaction between GB and HTPB, and the yield stress of the MPTMS@GB /HTPB suspension subsequently increases. However, when the MPTMS dosage increases to 0.1?g per 1 g GB, the MPTMS molecules covered on GB surface act as plasticizer to decrease the yield stress of the MPTMS@ GB/HTPB suspension.
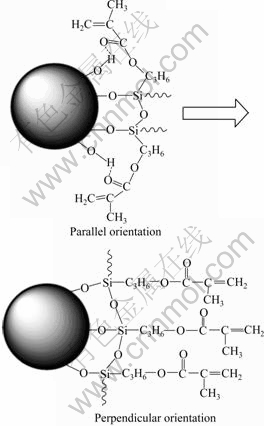
Fig.6 Orientation of CA molecules covered on GB surface
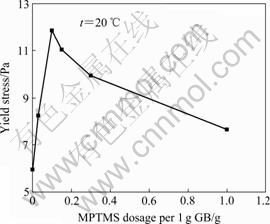
Fig.7 Variation of yield stress of MPTMS@GB/HTPB suspension containing 30% GB with MPTMS dosage
3.5 Effect of organofunctional group of CA on viscosity of GB/HTPB suspension
Fig.8 shows the rheological curves of MPTMS@ GB/HTPB and GPTES@GB/HTPB suspensions at 50?℃.
Apparently, the GPTES@GB/HTPB suspension behaves lower viscosity and weaker shear thinning than the MPTMS@GB/HTPB suspension in the range of the experiment shear rate. Based on the acid-base interaction theory[13], GPTES and MPTMS are Lewis acid and base, respectively. The acidic GPTES could efficiently enhance the dispersion of basic GB particles in the basic HTPB matrix, and weaken the inter-particulate interaction, which leads to the lower viscosity and weaker shear-thinning behavior of the suspension.
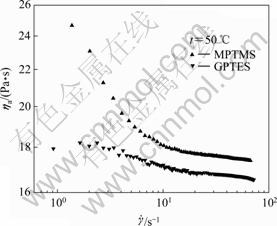
Fig.8 Rheological curves of MPTMS@GB/HTPB and GPTES@GB/HTPB suspensions (loading fraction: 50%; dosage of CA: 0.5?g per 1g GB)
4 Conclusions
1) The viscosity of the pristine GB/HTPB suspension increases with volume fraction of GB, and the relationship between its viscosity and volume fraction depends on the shear rate.
2) The modification of GB by MPTMS changes the viscosity of MPTMS@GB/HTPB suspension, and its viscosity is the minimum at the MPTMS dosage of 0.3?g per 1 g GB. Additionally, the modification of GB by MPTMS increases the yield stress of GB/HTPB suspension, and its yield stress is the maximum at the MPTMS dosage of 0.1?g per 1 g GB.
3) The GPTES@GB/HTPB suspension behaves lower viscosity and weaker shear thinning than the MPTMS@GB/HTPB suspension within the range of experimental shear rate.
References
[1] BARNES H A, HUTTON J F, WALTERS K. An introduction to rheology[M]. New York: Elsevier, 1989.
[2] POSLINSKI A J, RYAN M E, GUPTA R K, SESHADRI S G, FRELHETTE F J. Rheological behavior of filled polymeric system (Ⅰ): Yield stress and shear- thinning effects[J]. Journal of Rheology, 1988, 32(7): 703-735.
[3] METZNER A B. Rheology of suspensions in polymeric liquids[J]. Journal of Rheology, 1985, 29(6): 739-775.
[4] GUPTA R K, SESHADRI S G. Maximum loading levels in filled liquid systems[J]. Journal of Rheology, 1986, 30(3): 503-508.
[5] ZHOU Z W, SOLOMON M J, SCALES P J, BOGER D V. The yield stress of concentrated flocculated suspensions of size distributed particles[J]. Journal of Rheology, 1998, 43(3): 651-671.
[6] SHENOY A V. Rheology of filled polymer systems [M]. Dordrecht: Kluwer Academic Publishers, 1999.
[7] PROBSTEIN R F, SENGUN M Z, TSENG T C. Bimodal model of concentrated suspension viscosity for distributed particle sizes[J]. Journal of Rheology, 1994, 38(4): 811-829.
[8] KRIEGER I M, DOUGHERTY T J. A mechanism for the non-Newtonian in suspensions of rigid spheres[J]. Transactions of the Society of Rheology, 1959, 3(1): 137-152.
[9] MARON S H, PIERCE P E. Application of ree-eyring generalized flow theory to suspensions of spherical particles[J]. Journal of Colloid Science, 1956, 11(1): 80-95.
[10] KAWAGUCHI M, YAMAMOTO T, KATO T. Rheological studies of hydrophilic and hydrophobic silica suspensions in the presence of adsorbed poly(n-isopropylacrylamide)[J]. Langmuir, 1996, 12(26): 6184-6187.
[11] STAMHUIS J E, LOPP? J P A. Rheological determination of polymer-filler affinity[J]. Rheological Acta, 1982, 21(1): 103-105.
[12] MARY B, DUBOIS C, CARREAU P J, BROUSSEAU P. Rheological properties of suspensions of polyethylene-coated aluminum nanoparticles[J]. Rheological Acta, 2006, 45(5): 561-573.
[13] PLUEDDEMANN E P. Silane coupling agents[M]. New York: Plenum Press, 1991.
[14] LEE J D, YANG S M. Rheo-optical behaviors and stability of a silica particle suspension coated with silane coupling agents[J]. Journal of Colloid and Interface Science, 1998, 205(2): 397-409.
[15] XIE X L, TANG C Y, ZHOU X P, et al. Enhanced interfacial adhesion between PPO and glass beads in composites by surface modification of glass beads via in situ polymerization and copolymerization[J]. Chemistry of Materials, 2004, 16(1): 133-138.
[16] LIDDELL P V, BOGER D V. Yield stress measurements with the vane[J]. Journal of Non-Newtonian Fluid Mechanics, 1996, 63(2/3): 235-261.
[17] ZHOU Z W, SCALES P J, BOGER D V. Chemical and physical control of the rheology of concentrated metal oxide suspensions[J]. Chemical Engineering Science, 2001, 56(9): 2901-2920.
[18] ZAMAN A A, BJELOPAVLIC M, MOUDGIL B M. Effect of adsorbed polyethylene oxide on the rheology of colloidal silica suspensions[J]. Journal of Collloidal and Interface Science, 2000, 226(2): 290-298.
[19] POSTHUMUS W, MAGUSIN P C M M, BROKKEN-ZIJP J C M, TINNEMANS A H A, van der LINDE R. Surface modification of oxidic nanoparticles using 3-methacryl oxypropyltrimethoxysilane[J]. Journal of Collloidal and Interface Science, 2004, 269(1): 109-116.
[20] BAUER F, ERNST H, DECKER U, et al. Preparation of scratch and abrasion resistant polymeric nanocomposites by monomer grafting onto nanoparticles, 1 FTIR and multi-nuclear NMR spectroscopy to the characterization of methacryl grafting[J]. Macromolecular Chemistry and Physics, 2000, 201(18): 2654-2659.
(Edited by LONG Huai-zhong)
Received date: 2008-06-25; Accepted date: 2008-08-05
Corresponding author: XIE Xiao-lin, Professor, PhD; Tel: +86-27-87540053; E-mail: xlxie@mail.hust.edu.cn