以WO3-Al-C混合物为原料采用简易微波辅助法制备WC-Al2O3复合粉末
来源期刊:中国有色金属学报(英文版)2017年第12期
论文作者:A. KARIMZADEH BEHNAMI M. SAKAKI M. Sh. BAFGHI K. YANAGISAWA
文章页码:2630 - 2637
关键词:微波加热;燃烧合成;WC-Al2O3复合物
Key words:microwave heating; combustion synthesis; WC-Al2O3 composite
摘 要:提出一种操作简单、成本低廉的制备WC-Al2O3复合粉末的方法。热力学计算表明,WO3:xAl:(4-1.5x)C混合体系中WO3在铝热还原反应过程放出大量的热量,促进了此体系中的碳热还原反应(吸热反应)。用碳原子部分取代铝原子阻止了副产物W2C的形成,这是由于它在较低温度下具有较低的热力学稳定性。为验证本文作者所提出的反应机理,在微波炉中对不同WO3:xAl:(4-1.5x)C(1.1≤x≤2)混合物进行热处理。结果表明,当1.4≤x≤2时,反应类型为自蔓延高温合成反应,WO3:xAl:(4-1.5x)C混合体系中少量Al导致大量WC的产生;当混合体系中 x=1.4 mol时,体系经过自蔓延高温合成反应后,在密封环境下连续进行微波加热能获得不含W2C的WC-Al2O3复合物;当x≤1.3时,由于反应不完全以及剩余的反应物,上述反应变得缓慢。
Abstract: A facile and cheap method for the fabrication of WC-Al2O3 composite powder was proposed. Thermodynamic calculations indicate that aluminothermic reduction of WO3 in WO3:xAl:(4-1.5x)C mixture is accompanied by a great deal of heat, so carbothermic reduction reaction (endothermic) becomes activated in the system. By substitution of aluminum with carbon, the formation of unwanted W2C phase can be prevented because of less thermodynamic stability at lower temperatures. For the verification of the proposed reaction mechanism, various WO3:xAl:(4-1.5x)C mixtures (1.1≤x≤2) were heat-treated in a domestic microwave oven. The results showed that the type of reaction is self-propagating high temperature synthesis (SHS) when 1.4≤x≤2. Lower amount of Al in WO3:xAl:(4-1.5x)C mixtures results in a higher formation of WC. A W2C-free WC-Al2O3 composite was obtained by continuation of microwave heating in an isolated atmosphere, after the occurrence of SHS reaction in a mixture containing 1.4 mol of Al. Results also showed that when x≤1.3, type of reaction becomes gradual, which in turn brings about the incompleteness reaction and residue of initial reagents.
Trans. Nonferrous Met. Soc. China 27(2017) 2630-2637
A. KARIMZADEH BEHNAMI1, M. SAKAKI2,3, M. Sh. BAFGHI1,3, K. YANAGISAWA3
1. School of Metallurgy and Materials Engineering, Iran University of Science and Technology, Narmak, Tehran 16846-13114, Iran;
2. Department of Materials Engineering, Faculty of Engineering, Malayer University, Malayer 65719-95863, Iran;
3. Research Laboratory of Hydrothermal Chemistry, Kochi University, Kochi 780-8520, Japan
Received 7 October 2016; accepted 18 April 2017
Abstract: A facile and cheap method for the fabrication of WC-Al2O3 composite powder was proposed. Thermodynamic calculations indicate that aluminothermic reduction of WO3 in WO3:xAl:(4-1.5x)C mixture is accompanied by a great deal of heat, so carbothermic reduction reaction (endothermic) becomes activated in the system. By substitution of aluminum with carbon, the formation of unwanted W2C phase can be prevented because of less thermodynamic stability at lower temperatures. For the verification of the proposed reaction mechanism, various WO3:xAl:(4-1.5x)C mixtures (1.1≤x≤2) were heat-treated in a domestic microwave oven. The results showed that the type of reaction is self-propagating high temperature synthesis (SHS) when 1.4≤x≤2. Lower amount of Al in WO3:xAl:(4-1.5x)C mixtures results in a higher formation of WC. A W2C-free WC-Al2O3 composite was obtained by continuation of microwave heating in an isolated atmosphere, after the occurrence of SHS reaction in a mixture containing 1.4 mol of Al. Results also showed that when x≤1.3, type of reaction becomes gradual, which in turn brings about the incompleteness reaction and residue of initial reagents.
Key words: microwave heating; combustion synthesis; WC-Al2O3 composite
1 Introduction
WC-Al2O3 composites are useful materials for various industrial applications including production of drilling, cutting and machining tools as well as coating purposes. These composites show interesting properties such as low density, excellent hardness, good chemical inertness and high temperature strength [1-3].
Long time high temperature heating under a controlled atmosphere is the conventional method for the fabrication of WC/WC-Al2O3 composites, which makes the products considerably expensive [4,5]. Hence, various synthesis methods have been evaluated in order to find a simple route which shortens the reaction time and utilizes cheap reagents [6-9]. One of these methods is self-propagating high temperature synthesis (SHS).
SHS method is only applicable to the exothermic reactions. During this process, the heat released by chemical reactions propagates through the mixture of reagents spontaneously and facilitates the process to go forward [10,11]. According to Merzhanov’s criterion, when adiabatic temperature (The highest that a system could reach under an adiabatic condition, Tad) of an exothermic system is higher than 1800 K, the synthesis reaction type would be SHS [12]. SHS reactions could be initiated by: 1) a furnace, 2) an electrical element attached to the surface of the sample and 3) an igniter mixture. This synthesis method has some advantages such as high synthesis rate, in-complexity, low process cost and ability to produce pure compounds [11,13,14].
ZHANG et al [7] and NIYOMWAS [8] have reported the SHS synthesis of WC-Al2O3 powders by use of WO3-Al-C reagents. For this purpose, the SHS reaction was triggered by means of a tungsten wire. Their results showed that the product consists of unexpected W2C phase which has weak properties in comparison with WC. Several methods were used to decrease the amount of W2C compound in the product, such as use of mixture diluents (e.g., Al2O3 and NaCl) [8] and/or employment of active carbon as the carbon source [7]. Moreover, it has been proved that W2C phase could be leached from the product by use of a mixture of HNO3-HF acid [7].
Recently, activation of SHS reactions by microwave radiation has gained attention worldwide [15,16]. During microwave heating, interaction of electromagnetic wave and materials generates heat within the target. Thermal, magnetic and dielectric properties of the sample, as well as operating temperature, power and frequency of microwave influence the efficiency of heating process [17,18]. Since in microwave heating heat is generated within the materials, the temperature gradient is smaller than that of conventional heating routs; therefore, the product becomes more homogeneous [19]. Other benefits of use of microwave energy for heating are the decrease of production time and cost, ability of selective heating and less environmental pollution [20-22].
To the best of our knowledge, the first attempt on the microwave-assisted SHS synthesis of WC-Al2O3 composite powder is our work in Ref. [23]. It was found that the first reaction during microwave heating of WO3-Al-C mixture is the aluminothermic reduction of WO3. Tungsten carbide is subsequently obtained through the reaction of reduced tungsten and carbon. Our results showed that the product consists of W2C phase whose formation is supposed to be related to: 1) occurrence of carbon deficiency in the system during synthesis process, and 2) high thermodynamic stability of this phase at high temperatures resulted from the great deal of heat released by the reaction between WO3 and Al [23]. According to the mentioned reaction mechanism, formation of W2C phase could be prevented by A) adding extra amount of carbon to the initial WO3-Al-C mixture and B) decreasing the system temperature to make W2C phase thermodynamically unstable. Possibility of use of the first idea (idea A) for the production of W2C-free product has been investigated in our previous study [23]. It was found that although the increase of carbon amount in the system diminishes W2C phase, whole elimination of W2C phase is unreachable due to some technical shortages of the employed experimental setup [23].
The aim of the present study is to investigate the feasibility of use of idea B for the fabrication of WC-Al2O3 composite powder from WO3-Al-C mixture through microwave-assisted SHS process. This was achieved by substitution of some Al in reaction system with carbon. It is expected that lower Al content would decrease the maximum temperature obtained by aluminothermic reduction of WO3, which in turn decreases the amount of W2C phase in the product. For this purpose, various reagent mixtures were heated by means of a domestic microwave oven. Experimental observations together with the results obtained from XRD and SEM examinations were used to find the optimum synthesis conditions. Thermodynamic calculations were used to predict the possibility of probable reactions in the systems.
2 Experimental
The aim of this study is to find a proper route for the prevention of W2C phase formation during microwave heating of WO3-Al-C mixture, through decreasing the maximum temperature of the system. Possibility of occurrence of probable reactions, type of reactions (endo/exothermic) and adiabatic temperature of reactions (Tad) were estimated by use of 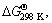
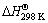 and Tad values, respectively. Thermodynamic calculations were performed by use of LibreOffice Calc software [24] and required thermodynamic data were obtained from Factsage website [25].
and Tad values, respectively. Thermodynamic calculations were performed by use of LibreOffice Calc software [24] and required thermodynamic data were obtained from Factsage website [25].
Analytical grade WO3, Al and C powders were used as reagents. Various amounts of starting materials were mixed in a low energy laboratory ball-mill with hardened steel balls and pot. Milling was performed at room temperature in air atmosphere. Milling parameters were as follows: rotation speed 250 r/min, ball-to-powder mass ratio 10, time 5 min. Green cylindrical samples, with a constant volume (10 mm in diameter and 4 mm in height, mass ~2 g), were obtained by using a single-axis hydraulic press with a pressure of 50 MPa.
A SAMSUNG (GE2370G) domestic microwave oven with an output power of 850 W was used for microwave heating. Our prior examinations showed that the microwave absorption capability of the green samples is insufficient for a successful reaction ignition. Therefore, a silicon carbide (SiC) block was placed under the samples to act as a susceptor and accelerate the heating [26,27]. Figure 1 illustrates a schematic of the experimental setup of this study.
Alumina blocks and powder as well as Pyrex beaker do not affect the heating process due to the high transparency of SiO2 and Al2O3 compounds to microwave radiation [19,26]. These materials were merely used in the setup to create an air isolated atmosphere for the samples.
The occurrence of SHS reaction was identified by observing the physical phenomena, such as flushing and inflaming of the samples during the heating process. It was observed that SHS reactions occur within 1 min of microwave heating. For mixtures with a gradual reaction mode, heating for 2 min was allowed.
After heating for appropriate time, microwave device was turned off and samples were allowed to cool down naturally. Obtained powders were analyzed by use of an X-ray diffraction instrument (XRD: JEOL-JDX8030 with a Cu Kα radiation). Microstructures of some powders were studied by means of a scanning electron microscope (SEM: TESCAN-VEGA II).
Fig. 1 Schematic illustration of experimental setup
3 Results and discussion
3.1 Thermodynamic aspects
Microwave heating is a non-equilibrium process, due to high heating/cooling rates. Thermodynamic calculations of non-equilibrium processes are not completely understood; nevertheless, using conventional thermodynamic calculations could give a useful general view about the probable reactions. The overall chemical reaction in WO3-Al-C mixture could be presented as
WO3+xAl+(4-1.5x)C=WC+(x/2)Al2O3+(3-1.5x)CO(g) (1)
In fact, Reaction (1) is the combination of the following sub-reactions:
WO3+2Al=W+Al2O3
(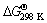 =-818 kJ,
=-818 kJ, 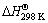 =-832 kJ) (2)
=-832 kJ) (2)
WO3+3C=W+3CO(g)
(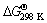 =352 kJ,
=352 kJ, 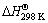 =511 kJ) (3)
=511 kJ) (3)
W+C=WC
(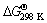 =-38 kJ,
=-38 kJ, 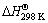 =-40 kJ) (4)
=-40 kJ) (4)
Aluminothermic reduction reaction of WO3 (Reaction (2)) is highly exothermic [6,28]; therefore, it is rational to assume that the released heat can probably activate the reaction between WO3 and C (Reaction (3)) in the WO3-Al-C mixture. Since Reaction (3) is endothermic [4], it is expected that simultaneous reduction of WO3 by Al and C would decrease the overall system temperature and can possibly prevent the W2C formation.
Table 1 shows 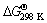 ,
, 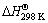 and Tad values of Reaction (1) for some Al amounts in the range of 1≤x≤2.
and Tad values of Reaction (1) for some Al amounts in the range of 1≤x≤2.
As shown in Table 1, for a mixture containing 2 mol of Al, standard free energy (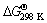 ) has a large negative value (-856 kJ), showing a high possibility and appetite for the reaction [29]. For this sample, the reaction is highly exothermic (
) has a large negative value (-856 kJ), showing a high possibility and appetite for the reaction [29]. For this sample, the reaction is highly exothermic (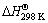 =-872 kJ), thus, the heat of the reaction could change the temperature of the bulk mixture. Our calculations (see Table 1) revealed that Tad of WO3:2Al:C mixture is about 3900 K, which is much higher than Merzhanov’s criterion [12]. Hence, from thermodynamic viewpoint, it is anticipated that a SHS reaction would occur during microwave heating of this mixture. As shown in Table 1, with decreasing the amount of Al in WO3:xAl:(4-1.5x)C mixture, values of
=-872 kJ), thus, the heat of the reaction could change the temperature of the bulk mixture. Our calculations (see Table 1) revealed that Tad of WO3:2Al:C mixture is about 3900 K, which is much higher than Merzhanov’s criterion [12]. Hence, from thermodynamic viewpoint, it is anticipated that a SHS reaction would occur during microwave heating of this mixture. As shown in Table 1, with decreasing the amount of Al in WO3:xAl:(4-1.5x)C mixture, values of 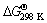 and
and 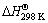 have increased, which means a lower reaction tendency and a lower exothermicity of the system. Lower exothermicity would bring about a lower adiabatic temperature.
have increased, which means a lower reaction tendency and a lower exothermicity of the system. Lower exothermicity would bring about a lower adiabatic temperature.
Table 1 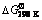 ,
, 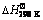 and Tad values of possible reactions in various WO3:xAl:(4-1.5x)C mixtures according to Reaction (1)
and Tad values of possible reactions in various WO3:xAl:(4-1.5x)C mixtures according to Reaction (1)
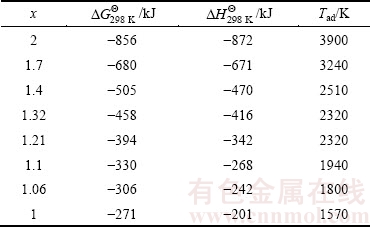
Regarding Table 1, the limit of Al amount by which exothermicity of Reaction (1) is high enough to meet the Merzhanov’s criterion, is around 1.06 mol. It is worth noticing that Tad has remained constant when 1.21≤x≤1.32. This is supposed to be due to the melting of Al2O3 in the product.
3.2 Experimental findings
Table 2 shows the composition of all investigated mixtures together with the types of occurred reactions. Types of reactions were identified by visual observation.
Table 2 Types of reactions in all investigated samples of this study (Physical phenomena, i.e., flushing and inflaming of samples, were considered as signs of SHS reactions)

When x=2, Reaction (1) would be as follows:
WO3+2Al+C=WC+Al2O3 (5)
Table 2 shows that the type of reaction for a WO3:2Al:C mixture (i.e., Reaction (5)) is SHS. This finding is in a good agreement with thermodynamic predictions as well as Merzhanov’s criterion. Figure 2 represents the XRD patterns of WO3:2Al:C mixture before and after microwave heating.
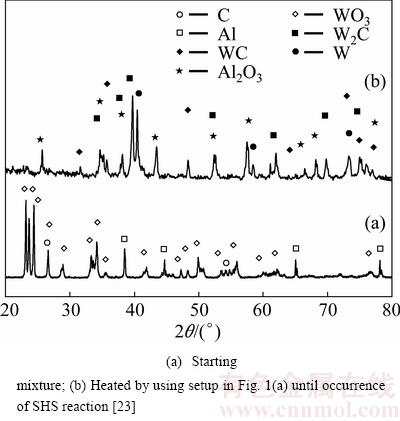
Fig. 2 XRD patterns of WO3:2Al:C system
As shown in Fig. 2, XRD pattern has completely changed due to the occurrence of a SHS reaction, and no peak of the starting reagents is visible in Fig. 2(b). The presence of alumina peaks reveals that reduction of WO3 by Al has occurred. Although merely formation of WC:Al2O3 compounds during microwave heating of WO3:2Al:C mixture is anticipated (see Reaction (5)), the product consists of unwanted W2C phase. Properties of W2C phase are inferior to those of WC; hence, its formation is undesirable. We have shown earlier that the presence of W2C phase is presumably due to 1) occurrence of carbon loss (see Reaction (6)) as the consequence of carbothermic reduction of WO3 [30] and/or 2) high thermodynamic stability of W2C compound at high obtained temperatures [6,7].
W+0.5C=0.5W2C
(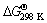 =-11 kJ,
=-11 kJ, 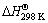 =-13 kJ) (6)
=-13 kJ) (6)
Both phenomena 1 and 2 are promoted by high temperatures resulted from intensively exothermic reduction reaction of WO3 by Al.
Based on the above justification, decrease of the systems maximum temperature to the extent that WC phase is thermodynamically stable is helpful for the production of WC-Al2O3 composite free from W2C compound. For this purpose, in this research work, the amount of C in the WO3:2Al:C system has been increased in expense of Al, in accordance with Reaction (1).
Figure 3 shows the XRD patterns of WO3:1.7Al: 1.45C sample before and after microwave heating.
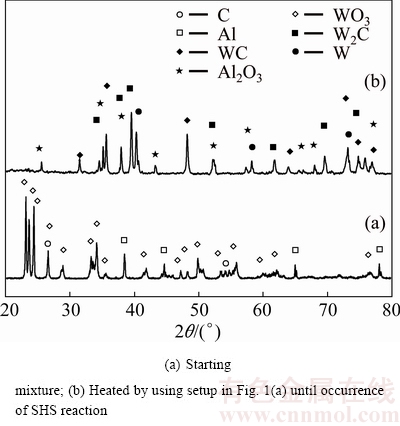
Fig. 3 XRD patterns of WO3:1.7Al:1.45C system
Abrupt change of XRD pattern together with the observation of ignition phenomenon (see Table 2) confirms the thermodynamic prediction and shows that the type of occurred reaction is SHS. Table 1 shows that by decreasing the amount of Al from 2 to 1.7 mol, calculated Tad of the system has decreased accordingly. Hence, a decrease in thermodynamic stability of W2C phase is anticipated. A comparison between Fig. 2(b) and Fig. 3(b) reveals that this prediction is true, so by lower amount of Al in the initial mixture, relative share of WC phase in the products has been increased. Figure 3(b) also shows no peaks of the starting reagents in the XRD pattern of the heated sample. Since the amount of Al in the WO3:1.7Al:1.45C mixture has not been sufficient for the reduction of entire WO3 in the mixture, the absence of WO3 peaks in Fig. 3(b) indicates that in this system, carbothermic reduction reaction has also occurred (Reaction (3)) alongside the aluminothermic reduction. Hence, the overall reaction could be written as follows:
WO3+1.7Al+1.45C=WC+0.885Al2O3+0.45CO(g) (7)
The presence of W2C peaks in Fig. 3(b) indicates that more Al decrease is necessary for better elimination of W2C phase from the product.
Figure 4 shows the XRD patterns of WO3:1.4Al: 1.9C sample before (Fig. 4(a)) and after (Fig. 4(b)) microwave heating.
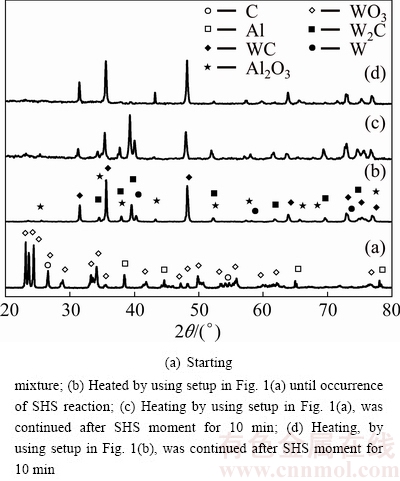
Fig. 4 XRD patterns of WO3:1.4Al:1.9C system
Sudden change of XRD pattern and observation of ignition phenomenon (see Table 2) show that the type of occurred reaction is SHS. As mentioned before, the absence of peaks of WO3 phase in Fig. 4(b) shows that WO3 is reduced by Al and C simultaneously, according to the following reaction:
WO3+1.4Al+1.9C=WC+0.7Al2O3+0.9CO(g) (8)
Table 1 shows that by decreasing Al from 1.7 to 1.4 mol, the calculated Tad has decreased accordingly which can promote the stability of WC [6,7]. The comparison of Figs. 2(b), 3(b) and 4(b) shows that this prediction is true and with less Al in the initial mixture, WC phase in the products has become more pronounced. However, peaks of W2C compound are still observed in Fig. 4(b). Hence, lower amount of Al should be used in the initial mixture.
XRD patterns of WO3:1.1Al:2.35C sample before and after microwave heating are presented in Fig. 5.
With regard to thermodynamic calculations (Table 1) the reaction in this system (Reaction (9)) is feasible and it is a SHS type.
WO3+1.1Al+2.35C=WC+0.55Al2O3+1.35CO(g) (9)
Figure 5(b) shows that peaks of the starting reagents remain in the XRD pattern of heated WO3:1.1Al:2.35C mixture. This phenomenon together with no sign of ignition during heating (see Table 2) indicates that despite thermodynamic predictions, the type of reaction has been gradual reaction. The change of type of reactions in WO3:xAl:(4-1.5x)C mixtures from SHS to gradual reaction, as a consequence of Al decrease, can also be concluded from SEM results. Figures 6(a) and (b) show SEM images of WO3:1.4Al:1.9C and WO3:1.1Al:2.35C mixtures, respectively.
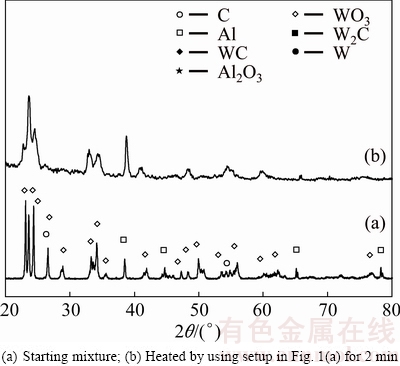
Fig. 5 XRD patterns of WO3:1.1Al:2.35C system
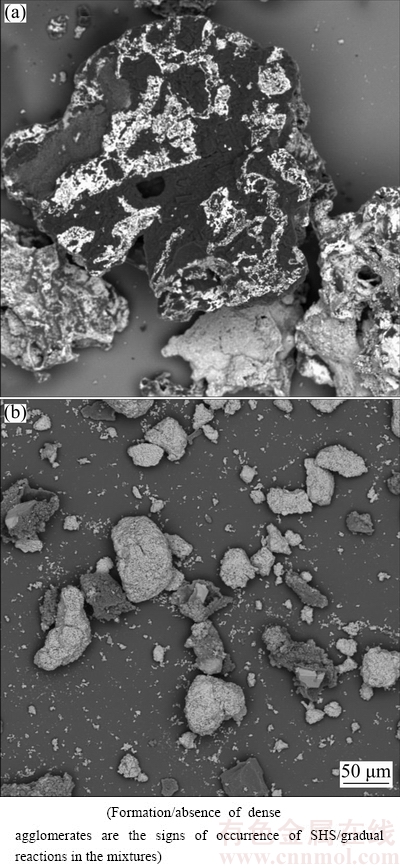
Fig. 6 BSE-SEM images of heated WO3:1.4Al:1.9C (a) and WO3:1.1Al:2.35C (b) mixtures
Figure 6 reveals that when the type of reaction is SHS (e.g., WO3:1.4Al:1.9C mixture), the product contains coarse and dense agglomerates due to partial fusion and coalescence of particles at high system temperatures; while due to low system temperatures during gradual reactions, there is no possibility for such phenomenon to take place and the particles remain separated (see Fig. 6(b)). The change of type of reaction from SHS to a gradual mode is supposed to be related to the fact that high amount of C in WO3:1.1Al:2.35C mixture decreases the contact between Al and WO3 particles, by its locating between Al and WO3. This, in turn, lowers the rate of aluminothermic reduction of WO3. Therefore, heat accumulation in the mixture will be diminished, which leads to the occurrence of a gradual reaction.
Our experimental results showed that the critical amount of Al in WO3:xAl:(4-1.5x)C mixture by which the type of reaction changes from SHS to gradual mode, is about 1.3 mol. The rate of material synthesis through gradual reactions is lower than that through SHS ones [10]; so longer time is required for the fabrication of the same final product. Since our experimental setup was not confidentially sealed against oxygen penetration into the reaction chamber for a long time, only SHS reactions were believed to be reliable. Therefore, a lower limit of x≥1.4 was set. With regard to Figs. 2(b), 3(b) and 4(b), the highest amount of WC phase is obtained with a WO3:1.4Al:1.9C mixture. However, some amount of unwanted W2C phase is observed in the product (see Fig. 4(b)). Further elimination of W2C was expected to be possible by continuation of microwave heating after the occurrence of SHS reaction, as it was proposed in our previous work [23].
The XRD pattern of WO3:1.4Al:1.9C mixture heated for 10 min is shown in Fig. 4(c). This pattern shows that by heating continuation, amounts of W and W2C phases have increased. This phenomenon could possibly be attributed to the penetration of some amount of oxygen into the reaction chamber which could decompose WC phase according to the following reactions:
WC+0.5O2(g)=W+CO(g)
(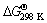 =-99 kJ,
=-99 kJ, 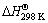 =-70 kJ) (10)
=-70 kJ) (10)
WC+O2(g)=W+CO2(g)
(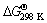 =-356 kJ,
=-356 kJ, 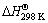 =-353 kJ) (11)
=-353 kJ) (11)
WC+0.25O2(g)=0.5W2C+0.5CO(g)
(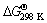 =-41 kJ,
=-41 kJ, 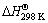 =-28 kJ) (12)
=-28 kJ) (12)
WC+0.5O2(g)=0.5W2C+0.5CO2(g)
(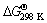 =-170 kJ,
=-170 kJ, 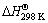 =-170 kJ) (13)
=-170 kJ) (13)
In order to increase the protection efficiency of the experimental setup against air penetration, the following modifications were made:
1) Sufficient amount of alumina powder was added into the inner space of Pyrex beaker (see Fig. 1(b)) to act as an air penetration barrier.
2) Additional amount of carbon (30%, mole fraction) was added to the WO3:1.4Al:1.9C mixture. So, when some oxygen (as air) passes through the alumina layer, it will react with the additionally used carbon.
XRD pattern of WO3:1.4Al:1.9C mixture heated for 10 min by employment of the modified setup, is shown in pattern Fig. 4(d). The comparison of Figs. 4(b) and (d) reveals that in the latter case, WC-Al2O3 composite powder has been synthesized successfully. This shows that use of alumina barrier layer together with additional carbon amount in the mixture provides a good solution to the oxygen penetration problem, so W and W2C phases (see Fig. 4(b)) can convert to WC through Reactions (4) and (14), respectively.
0.5W2C+0.5C=WC
(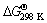 =-27 kJ,
=-27 kJ, 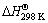 =-27 kJ) (14)
=-27 kJ) (14)
4 Conclusions
1) The amount of Al (x) in the WO3:xAl:(4-1.5x)C mixture has a considerable effect on the reaction tendency for occurrence (ΔGΘ), reaction exothermicity (ΔHΘ) and Tad values of the system.
2) When 1.4≤x≤2, the type of reaction is found to be SHS. It is also found that at x≤1.3, the reaction type changes to a gradual mode, which brings about incompleteness of reaction and remaining some initial reagents in the product.
3) The occurrence of SHS/gradual reaction can be recognized by observation of the presence/absence of large and dense agglomerates in the heated products, which is an indirect scale of the system heat release as well as the reaction type, consequently.
4) For mixtures with a SHS type (1.4≤x≤2) reaction, the products would consist of unwanted W2C phase and WO3 is reduced by Al and C simultaneously. By decreasing the system temperature through partial substitution of Al with C, the amount of W2C phase in the product decreases considerably.
5) Although in WO3:1.4Al:1.9C mixture, WC is the main obtained phase, W2C phase is also found in the product. A complete elimination of W2C phase can be achieved by continuation of microwave heating after the occurrence of SHS reaction, together with applying necessary modification to the experimental setup for confidential prevention of oxygen penetration into the reaction chamber.
6) A good consistency was found between the thermodynamic predictions and experimental results.
References
[1] ZHENG D, LI X, AI X, YANG C, LI Y. Bulk WC-Al2O3 composites prepared by spark plasma sintering [J]. International Journal of Refractory Metals and Hard Materials, 2012, 30: 51-56.
[2] QU H, ZHU S, DI P, OUYANG C, LI Q. Microstructure and mechanical properties of WC-40vol%Al2O3 composites hot pressed with MgO and CeO2 additives [J]. Ceramics International, 2013, 39: 1931-1942.
[3] QU H, ZHU S. Two step hot pressing sintering of dense fine grained WC-Al2O3 composites [J]. Ceramics International, 2013, 39: 5415-5425.
[4] SCHWARZKOPF P, KIEFFER R. Cemented carbides [M]. New York: Macmillan, 1986.
[5]  H. Alumina: Processing, properties, and applications [M]. Berlin: Springer, 1984.
H. Alumina: Processing, properties, and applications [M]. Berlin: Springer, 1984.
[6] SAKAKI M, BAFGHI M S, VAHDATI KHAKI J, ZHANG Q, SAITO F. Conversion of W2C to WC phase during mechano- chemical synthesis of nano-size WC-Al2O3 powder using WO3-2Al-(1+x)C mixtures [J]. International Journal of Refractory Metals and Hard Materials, 2013, 36: 116-121.
[7] ZHANG J, LEE J H, WON C W, CHO S S, CHUN B S. Synthesis of Al2O3-WC composite powder by SHS process [J]. Journal of Materials Science, 1999, 34: 5211-5214.
[8] NIYOMWAS S. The effect of diluents on synthesis of alumina–tungsten carbide composites by self-propagating high temperature synthesis process [C]//Proceedings of the Technology and Innovation for Sustainable Development International Conference (TISD2010). Nongkhai, Thailand, 2010: 1025-1029.
[9] HAN B Q, XU J L, LI N. Formation of Al2O3-WC powders from Al-WO3-C mixtures [J]. Mineral Processing and Extractive Metallurgy, 2006, 115: 189-194.
[10] MOORE J J, FENG H J. Combustion synthesis of advanced materials: Part I. Reaction parameters [J]. Progress in Materials Science, 1995, 39: 243-273.
[11] LIU G, LI J, CHEN K. Combustion synthesis of refractory and hard materials: A review [J]. International Journal of Refractory Metals and Hard Materials, 2013, 39: 90-102.
[12] NOVIKOV N P, BOROVINSKAYA I P, MERZHANOV A G. Thermodynamic analysis of reactions of self-propagating high-temperature synthesis [C]//Combustion Processes in Chemical Technology and Metallurgy. Chernogolovka: OIKHF AN SSSR, 1975: 174-178.
[13] DOU Z H, ZHANG T A, ZHANG Z Q, ZHANG H B, HE J C. Preparation and characterization of LaB6 ultra fine powder by combustion synthesis [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1790-1794.
[14] LI Y, LIU M. Gas sensing properties of Y-doped ZnO nanosheets synthesized via combustion method [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 2247-2252.
[15] MOUSAVIAN R T, SHARAFI S, SHARIAT M H. Microwave-assisted combustion synthesis in a mechanically activated Al-TiO2-H3BO3 system [J]. International Journal of Refractory Metals and Hard Materials, 2011, 29: 281-288.
[16] ATONG D, CLARK D E. Ignition behavior and characteristics of microwave-combustion synthesized Al2O3-TiC powders [J]. Ceramics International, 2004, 30: 1909-1912.
[17] ZHOU S C, BAI C G. Microwave direct synthesis and thermoelectric properties of Mg2Si by solid-state reaction [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1785-1789.
[18] ZHU J Y, ZHANG J X, ZHOU H E, QIN W Q, CHAI L Y. Microwave-assisted synthesis and characterization of ZnO-nanorod arrays [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 1578-1582.
[19] GANESH I, JOHNSON R, RAO G V N, MAHAJAN Y R, MADAVENDRA S S, REDDY B M. Microwave-assisted combustion synthesis of nanocrystalline MgAl2O4 spinel powder [J]. Ceramics International, 2005, 31: 67-74.
[20] JOSHI S C, BHUDOLIA S K. Microwave–thermal technique for energy and time efficient curing of carbon fiber reinforced polymer prepreg composites [J]. Journal of Composite Materials, 2014, 48: 3035-3048.
[21] RAJABI A, AIENERAVAIE M, DOROSTI V, SADRNEZHAAD S K. Development and biomedical application of nanocomposites: In situ fabrication of ZnO-PbO nanocomposite through microwave method [J]. Materials Technology, 2014, 29: 227-231.
[22] SEYEDNEZHAD M, RAJABI A, MUCHTAR A, RAO SOMALU M. Nanostructured and nonsymmetrical NiO-SDC/SDC composite anode performance via a microwave-assisted route for intermediate-temperature solid oxide fuel cells [J]. Materials and Manufacturing Processes, 2016, 31: 1301-1305.
[23] SAKAKI M, KARIMZADEH BEHNAMI A, BAFGHI M S. An investigation of the fabrication of tungsten carbide–alumina composite powder from WO3, Al and C reactants through microwave-assisted SHS process [J]. International Journal of Refractory Metals and Hard Materials, 2014, 44: 142-147.
[24] LibreOffice Project (Free Office Suite) [EB/OL] [2017-04]. http://www.libreoffice.org.
[25] FactSage thermo chemical software and database [EB/OL] [2017-04]. http://www.factsage.com.
[26] THOSTENSON E T, CHOU T W. Microwave processing: Fundamentals and applications [J]. Composites (Part A): Applied Science and Manufacturing, 1999, 30: 1055-1071.
[27] GROSSIN D, MARINEL S, NOUDEM J G. Materials processed by indirect microwave heating in a single-mode cavity [J]. Ceramics International, 2006, 32: 911-915.
[28] SAKAKI M, BAFGHI M S, KHAKI J V, ZHANG Q, KANO J, SAITO F. Effect of the aluminum content on the behavior of mechanochemical reactions in the WO3-C-Al system [J]. Journal of Alloys and Compounds, 2009, 480: 824-829.
[29] GASKELL D R. Introduction to the thermodynamics of materials [M]. New York: Taylor & Francis, 2003.
[30] LASSNER E, SCHUBERT W D. Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds [M]. New York: Kluwer Academic/Plenum Publishers, 1999.
A. KARIMZADEH BEHNAMI1, M. SAKAKI2,3, M. Sh. BAFGHI1,3, K. YANAGISAWA3
1. School of Metallurgy and Materials Engineering, Iran University of Science and Technology, Narmak, Tehran 16846-13114, Iran;
2. Department of Materials Engineering, Faculty of Engineering, Malayer University, Malayer 65719-95863, Iran;
3. Research Laboratory of Hydrothermal Chemistry, Kochi University, Kochi 780-8520, Japan
摘 要:提出一种操作简单、成本低廉的制备WC-Al2O3复合粉末的方法。热力学计算表明,WO3:xAl:(4-1.5x)C混合体系中WO3在铝热还原反应过程放出大量的热量,促进了此体系中的碳热还原反应(吸热反应)。用碳原子部分取代铝原子阻止了副产物W2C的形成,这是由于它在较低温度下具有较低的热力学稳定性。为验证本文作者所提出的反应机理,在微波炉中对不同WO3:xAl:(4-1.5x)C(1.1≤x≤2)混合物进行热处理。结果表明,当1.4≤x≤2时,反应类型为自蔓延高温合成反应,WO3:xAl:(4-1.5x)C混合体系中少量Al导致大量WC的产生;当混合体系中 x=1.4 mol时,体系经过自蔓延高温合成反应后,在密封环境下连续进行微波加热能获得不含W2C的WC-Al2O3复合物;当x≤1.3时,由于反应不完全以及剩余的反应物,上述反应变得缓慢。
关键词:微波加热;燃烧合成;WC-Al2O3复合物
(Edited by Wei-ping CHEN)
Corresponding author: M. SAKAKI; Tel: +98-851-2232346; Fax: +98-851-2221977; E-mail: masoudsakaki79@gmail.com; masoud_sakaki@iust.ac.ir
DOI: 10.1016/S1003-6326(17)60291-7

