Article ID: 1003-6326(2005)02-0247-05
Fabrication of finegrained Al2O3 ceramic at low sintering temperature
LI Hua-ling(李华玲)1, 2, WANG Mao-cai(王茂才)1,
ZHAO Ji-bin(赵吉宾)3, LIU Wei-jun(刘伟军)3
(1. State Key Laboratory for Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China;
2. Graduate School of Chinese Academy of Sciences, Beijing 100039, China;
3. Shenyang Institute of Automation, Chinese Academy of Sciences, Shenyang 110016, China)
Abstract: A research on fabrication of finegrained Al2O3 ceramic at lower sintering temperature was carried out. Al2O3 powder with 50nm in diameter is compounded with 11.24%Al and 4.75%Fe(mass fraction) by high-energy ball-milling. Al is got from Al powder which is a component of the materials being milled and Fe from steel milling balls and milling jar during the milling. In this way, nearly no impurity is brought into the composite powder during milling. With hot pressing of the composite powder and pure Al2O3 powder, it is proved that Al2O3 powder can be densified at lower sintering temperature when the powder is compounded in this way. Al2OC and AlFe form during sintering process of the composite powder. With the reactive sintering and multiphase sintering mechanisms, finegrained Al2O3 ceramic is fabricated at low sintering temperature.
Key words: finegrained Al2O3 ceramic; sintering; nanometer Al2O3-Al-Fe composite powder; high-energy ball-milling; strength; toughness CLC number: TF124.5; TF123.7
Document code: A
1 INTRODUCTION
Al2O3 ceramic has many super properties. It is widely used in many fields[1]. While usually the sintering temperature of it is very high(about 1550-1950℃). Al2O3 grains grow rapidly at so high temperature, which will make the mechanical properties of the ceramic, especially toughness and strength, decrease. So a considerable effort has been made on how to fabricate dense finegrained Al2O3 ceramic at lower sintering temperature[2-4]. But until now, this problem hasnt been solved very well. Multiphase sintering is a popular technique that has been developed on this. Some phases, such as MgO, SiC, Y2O3 and AlFex, have been found to be good second phases for the sintering. But usually people just mix these powders into Al2O3 powder and sinter them[5-8]. Some impurities usually are brought into the powders in the process of mixing[9], and the sintering result cant satisfy them enough. In order to solve this problem, Al2O3 powder and Al powder were milled with steel milling balls in steel milling jar using high-energy ball-mill here. Then some amount of Fe that broke away from milling balls and milling jar came into the powders being milled and nanometer Al2O3-11.24%Al-4.75%Fe(mass fraction) composite powder was prepared. In this way, nearly no impurity was brought into the composite powder. The intermetallic compound, AlFex, was expected to form when the composite powder was sintered. Then reactive sintering and multiphase sintering mechanisms would give effect to the sintering process at the same time, which could accelerate the sintering rate and be beneficial to fabricating finegrained Al2O3 ceramic.
2 EXPERIMENTAL
The Al2O3 powder used here has an average particle size of 50nm and the industrial aluminium powder of 75-150μm. In preparing the Al2O3-Al-Fe composite powder, SPEX 8000 high-energy ball-mill with rotation speed of 875r/min, steel milling jar and milling balls were used. The mass ratio of ball to powder for the milling was 10∶1 and the milling time was 13.5h. After the milling, the final compositions of the powder were Al2O3-11.24%Al-4.75%Fe(mass fraction). The ferrum came from the milling balls and milling jar. The pure Al2O3 powder and the composite powder were put into die respectively without cold pressing and sintered. Three samples were obtained. The sintering conditions for the samples are listed in Table 1. The powder morphologies were observed with PHILIPS EM420 TEM and the impact fracture microstructures of three samples with PHILIPS XL30 SEM. Japanese D/max-2500PC XRD was used to identify the phases of the powders and the samples. The densities of the samples were determined using Archimedes method.
Table 1 Sintering conditions for samples
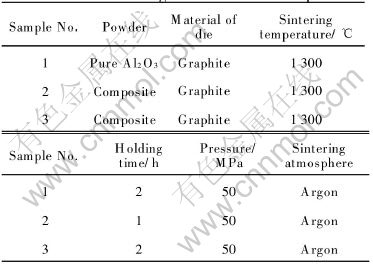
3 RESULTS AND DISCUSSION
3.1 TEM research
The morphologies of the pure Al2O3 and the Al2O3-Al-Fe powder being milled for different times are presented in Figs.1(a)-(f) respectively. From these images we can see that, during the high-energy ball-milling, Al2O3 particulates wedge themselves into Al particulates first. As milling goes on, the composite powder is homogenized and smashed. Fe particulate isnt seen in the images. The possible reason is that Fe particulates are very fine when they break away from milling jar and milling balls. They come into Al particulates too during the milling. Finally, fine homogeneous Al2O3-Al-Fe composite powder is obtained.
3.2 XRD research
XRD pattern of the composite powder is shown in Fig.2(a), from which, the components of the composite powder obtained are confined to be Al2O3, Al and Fe. Using the XRD technique, the phases of sample 2 and sample 3 are revealed to be the same: Al2O3+AlFe+Al2OC. The XRD pattern of sample 3 is shown in Fig.2(b). Fig.2(a) shows that no phase transformation and reaction about aluminium and alumina occur during the milling process. The reactions occuring upon sintering are as follows:
Al+Fe→AlFe(1)
Al+ Al2O3+C→Al2OC(2)
where the carbon mainly comes from the graphite dies.
3.3 Density
The relative densities of sample 1, sample 2 and sample 3 are determined to be 73%, 79% and 93%, respectively.
3.4 SEM research
Figs.3(a), (b) and (c) present the fractured surface images of samples 1, 2 and 3, respectively. As presented in Fig.3(a), there are many pores with tens to hundreds nanometers in diameter in sample 1 and no regular polyhedron Al2O3 grain can be seen in it.
There are many pores in sample 2 too, but exact Al2O3 grain morphology cant be seen in its fractured surface because the grain surface is covered with many whiskers with about 100-200nm in length and many particulates with about tens of nanometers in diameter distributing among the whiskers, which can be seen in Fig.3(b). According to the XRD pattern (Fig.2(b)) and the morphology, the whiskers are confirmed to be Al2OC and the particulates to be AlFe. Some reactions about Al and Fe occur and Al2OC and AlFe form during sintering.
Fig.3(c) shows the fractured surface image of sample 3, from which sample 3 is found to be much denser than the other samples and the Al2O3 grain morphology in it to be regular polyhedron. The microstructure of the sample is mixed type of intergranular and intragranular fracture. Al2OC whiskers with 100-400nm in length locate interlacedly at Al2O3 grain boundaries (as shown by arrow 1 in Fig.3(c)). The whiskers are the ones that the Al2OC grains in sample 2 grow up to be. The addition of Al2OC can increase the material toughness and strength. The toughening and strengthening mechanisms are mainly interface debonding, crack deflection and whisker pullout. In the sample, AlFe grains with about tens of nanometers in diameter can be seen in some Al2O3 grains or at grain boundaries (as shown by arrow 2 in Fig.3(c)). The particulates can improve the toughness of the material too. The toughening mechanisms are mainly interface debonding and crack deflection. The chemical combination about Al2OC and AlFe releases energy which accelerates Al2O3 grain boundary diffusion and bulk diffusion and increases densification rate.
Some formation models about AlFe and Al2OC in the material are conceived as follows. After the high-energy ball-milling, Al2O3 particulates are coated with a certain amount of Al and Fe. When the composite powder is sintered, AlFe and Al2OC form on the surface of Al2O3 particulates. Al2OC whisker is a long-cycle modulation crystal and composed of Al2O3 and Al4C3, which connects with Al2O3 grain tightly in the material. The strength of Al2OC is high. It can hinder crack in its extending[10]. With this phase in the ceramic, the toughness and strength of the material can be increased. At the beginning of sintering, tiny AlFe particulates and Al2OC whiskers exist on the surface of Al2O3 particulates. As sintering keeps on, Al2OC whiskers and AlFe particulates can grow up through Al2O3 grain boundary diffusion or surface diffusion.
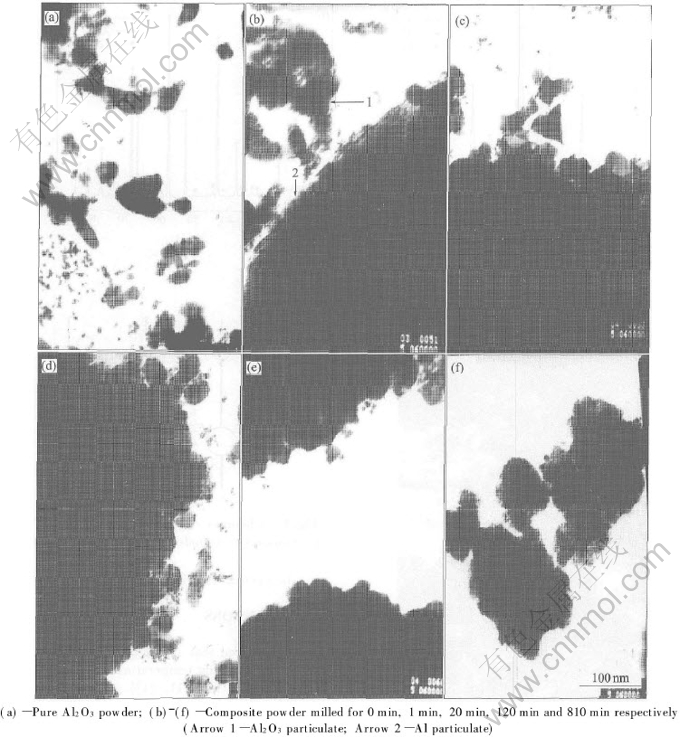
Fig.1 TEM images of powders
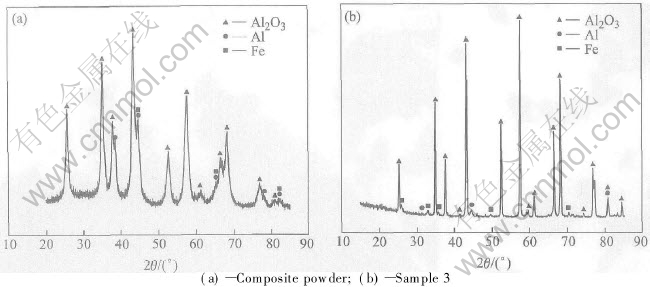
Fig.2 XRD patterns of samples

Fig.3 Fracture SEM images of samples
The growth process of Al2O3 grains is the transfer process of Al2O3 grain boundaries, so only when the transfer driving force is higher than the resistance of AlFe particulates and Al2OC whiskers at Al2O3 grain boundaries can the Al2O3 grains grow up. The maximal resistance, Fmax, that AlFe particulate can give to the transfer of Al2O3 grain boundary, is approximately expressed as[11]
Fmax=πrγb(3)
where r is the radius of AlFe particulate; γb is the Al2O3 grain boundary energy per unit area.
So, as shown in Fig.4, only the AlFe particulates small enough can be passed by Al2O3 grain boundary and come into Al2O3 grain and the bigger particulates are kept at the boundary. AlFe particulate in Al2O3 grain decreases the strength of Al2O3 grain and is beneficial to the happening of intragranular fracture, which improves the mechanical properties of the material[12-14]. Al2OC whisker has high length-to-diameter ratio. It gives higher resistance to the transfer of Al2O3 grain boundary than AlFe particulate. Al2OC whisker can’t be passed. It is kept at the boundary.
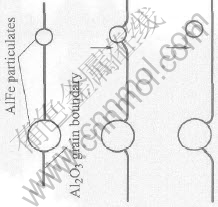
Fig.4 Schematic diagram of resistance of AlFe particulates to transfer of Al2O3 grain boundary
4 CONCLUSIONS
It is proved that Al2O3 powder can be densified at low sintering temperature when the powder is compounded with 11.24%Al and 4.75%Fe(mass fraction) by high-energy ball-milling. In the Al2O3-Al-Fe composite powder, Fe comes from steel milling balls and milling jar during the milling and Al from Al powder, a component of the materials being milled. In this way, nearly no impurity is brought into the composite powder during milling. Al2OC and AlFe form during the sintering process of the composite powder. With the reactive sintering and multiphase sintering mechanisms, finegrained Al2O3 ceramic is fabricated at lower sintering temperature.
REFERENCES
[1]JIN Zhi-hao, GAO Ji-qiang, QIAO Guan-jun. Engineering Ceramic Materials [M]. Xian: Xian Jiaotong University Press, 2000. 134-137. (in Chinese)
[2]YIN Sheng. Modern Ceramics and Their Applications [M]. Beijing: Beijing Science and Technology Press, 1990. 65. (in Chinese)
[3]GAO Lian, LI Wei. Nanometer Ceramics [M]. Beijing: Chemistry Industry Press, 2002. 83-113. (in Chinese)
[4]ZHANG Li-de, MOU Ji-mei. Nano-materials and Nano-structure [M]. Beijing: Science Press, 2001. 173. (in Chinese)
[5]FAN En-rong. Fabrication of Al2O3 ceramic at low sintering temperature [J]. Advanced Ceramics, 1996, 2: 42-45. (in Chinese)
[6]LIU Fu-tian, CHANG Jun, WANG Zhi, et al. Reactive sintering technique about ZrO2-3Al2O3·2SiO2-Al2O3/SiCn nano-micron-multiphase ceramics technique by in-situ reactive sintering [J]. Ceramics Engineering, 2001, 10: 35-38. (in Chinese)
[7]HUO Zhen-wu, SI Wen-jie, LIU Da-peng, et al. Influence of doping on the densification rate of effect of high-purity alumina ceramics [J]. Bulletin of the Chinese Ceramic Society, 2002, 2: 8-11. (in Chinese)
[8]YIN Yan-sheng, LI Jia, SUN Kang-ning, et al. Fe-Al/ Al2O3 composites: research and application [J]. Materials Review, 2003, 17(2): 73-75. (in Chinese)
[9]LIU Yin, WANG Jing, ZHANG Ming-xu, et al. Research and development of mechanical attrition method in nanostructural materials [J]. Materials Review, 2003, 17(7): 20-22. (in Chinese)
[10]YIN Yan-sheng, ZHANG Jing-de. Alumina Ceramic and Alumina-based Composite Materials [M]. Beijing: Chemistry Industry Press, 2001. 191-195. (in Chinese)
[11]HOU Zeng-shou, LU Guang-xi. Metallurgy Theories [M]. Shanghai: Shanghai Science and Technology Press, 1990. 206-207. (in Chinese)
[12]Niihara K. New design concepts of structural ceramics-ceramic nanocomposites [J]. J Ceram Soc Jpn, 1991, 99(10): 974-982.
[13]Piciacchio A, Lee S H, Messing G L. Processing and microstructure development in alumina-silicon carbide intragranular particulate composites [J]. J Am Ceram Soc, 1994, 77(8): 2157-2164.
[14]HOU Yao-yong, LI Li, ZHANG Ju-xian. Study on the microstructure and strength-toughening mechanisms of Al2O3/SiC nanocomposites [J]. J Chin Electron Micros Soc, 1998, 17(2): 156-161. (in Chinese)
Foundation item: Project(2001AA421160) supported by the Hi-tech Research and Development Program of China
Received date: 2004-12-06; Accepted date: 2005-01-18
Correspondence: WANG Mao-cai, Professor; E-mail: wmc@imr.ac.cn
(Edited by YANG Bing)