J. Cent. South Univ. Technol. (2009) 16: 0101-0107
DOI: 10.1007/s11771-009-0017-3
Removal of Cr(Ⅲ) and Cr(Ⅵ) from aqueous solution by adsorption on sugarcane pulp residue
YANG Zhi-hui (杨志辉), WANG Bing(王 兵), CHAI Li-yuan(柴立元), WANG Yun-yan(王云燕),
WANG Hai-ying(王海鹰), SU Chang-qing(苏长青)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Sugarcane pulp residue (SPR), a waste from sugar-refinery, which possesses a large surface area, can be used for removing chromium (Cr(Ⅲ) and Cr(Ⅵ)) from wastewater. In this work, the kinetics, isotherms of Cr(Ⅲ) and Cr(Ⅵ) adsorption and their removal by SPR were investigated. The results show that the removal percentages of Cr(Ⅵ) and Cr(Ⅲ) increase with increasing SPR dosage and temperature and decrease with increasing SPR particle size and the initial concentration of chromium ions. However, the influence of pH value on the Cr(Ⅵ) removal differs from that of the Cr(Ⅲ) removal. The Cr(Ⅵ) removal percentage decreases with increasing pH values, while the Cr(Ⅲ) removal percentage increases with increasing pH value. The adsorption kinetics of Cr(Ⅵ) and Cr(Ⅲ) well fits with pseudo-second-order model. Langmuir adsorption isotherm can well describe the adsorption phenomena of chromium ions with the maximum adsorption capacity of 0.567 mg/g for Cr(Ⅵ) and 3.446 mg/g for Cr(Ⅲ). Moreover, SPR reveals higher adsorption capacity for Cr(Ⅲ) than that for Cr(Ⅵ), which implies that SPR has more potential application for Cr(III)-containing wastewater treatment than that for Cr(Ⅵ)-containing wastewater treatment.
Key words: Cr(Ⅵ); Cr(Ⅲ); removal; adsorption; sugarcane pulp residue
1 Introduction
In recent years, chromium compounds have been widely used in many modern industries such as metal finishing, leather tanning, electroplating, pigment manufacture and combustion of coal and oil, resulting in environment contamination. In particular, hexavalent chromium has become a major concern[1-2]. Therefore, the removal of chromium from wastewater arouses great attention. Several methods of chromium removal including chemical precipitation, reverse osmosis, ion exchange, foam flotation and electrolysis have been reported, but most of them have disadvantages such as high cost, large input of chemicals and incomplete removal[3-4].
The use of an adsorption process in the treatment of wastewater containing toxic metal ions is an attractive and suitable technique[5-6]. A variety of materials are used as adsorbents for chromium removal, and various studies have been published, documenting its adsorption on activated carbon[7], hydrotalcite[8], coconut husk[9], maple sawdust[10], biogas residual slurry[11], cellulosic graft copolymers[12], cross-linked chitosan[13], lignin [14] and red mud[15].
In China, the planting area of sugarcane accounts for 15×109 m2, and sugarcane yield reaches up to 998 Mt. About 112 Mt of sugarcane pulp residue (SPR) is discharged from sugar manufactory annually, which occupies a large area for its deposition. Moreover, the discharge of residue from agricultural by-product will cause an environment problem. Therefore, it is essential to utilize this residue to eliminate environmental and limited land stresses in China. SPR contains a large proportion of cellouse in possession of huge surface area. It was used to remove Ca2+, Ni2+, Zn2+ and Cu2+ from waste water[16]. However, the research on the removal of chromium ions by SPR is rather scanty. In industrial wastewater, hexavalent chromium and trivalent chromium simultaneously exist in some specific environmental conditions. Moreover, trivalent chromium can be converted into toxic hexavalent chromium. Therefore, the aim of the present investigation is to evaluate the removal of Cr(VI) and Cr(III) in aqueous solution by adsorption on sugarcane pulp residue, and to quantify the effect of pH, temperature, chromium concentration, SPR dosage and SPR particle size on the removal of Cr(VI) and Cr(III).
2 Experimental
2.1 Sugarcane pulp residue
The SPR used in this study was collected from a sugar-refinery in Hunan Province, China. It was sun-dried and ground to pass a 0.25 mm sieve prior to use.
2.2 Adsorption of Cr(Ⅲ) and Cr(Ⅵ)
The stock solutions of 1 g/L Cr(Ⅲ) and Cr(Ⅵ) were prepared by dissolving 5.1250 g CrCl3·6H2O and 2.833 0 g K2Cr2O7 in 1 L distilled water, respectively. 0.5 g SPR and a known initial concentration of Cr(Ⅲ) or Cr(Ⅵ) in a total volume of 15 mL were placed into a 100 mL polyethylene plastic bottle. The pH values of the solutions were adjusted to the desired values with either HCl or NaOH solution. The samples were shaken for 24 h at room temperature. After reaching the adsorption equilibrium, the adsorbent was removed by filtration. One portion of filtrate was taken for measuring the total chromium concentration by flame atomic adsorption spectrophotometer. The other portion of the filtrate was taken for determining Cr(Ⅵ) concentration with diphenylcarbazide colorimetric method. Cr(Ⅲ) concentration was calculated from the difference between the total chromium and Cr(Ⅵ) concentration in the filtrate. The pH values were measured by a pH meter (LeiCi, Shanghai PHS-3s with B-201-C composite electrode). In this work, the Cr(Ⅲ) and Cr(Ⅵ) removal affected by pH value, temperature, the initial Cr(Ⅲ) and Cr(Ⅵ) concentrations, and the particle size of SPR was studied.
2.3 Kinetics of Cr(Ⅲ) and Cr(Ⅵ) adsorption
0.5 g SPR was put into a 100 mL polyethylene plastic bottle containing a definite volume (15 mL in each case) of solution with 200 mg/L Cr(Ⅲ) and 50 mg/L Cr(Ⅵ), respectively. At room temperature, the polyethylene plastic bottles were placed on a shaker. At a predefined interval of time, the test solutions were filtrated to separate the adsorbent material, and the supernatant was chosen for determining the concentrations of total chromium and Cr(Ⅵ).
2.4 Characteristics of sugar pulp residue
The surface structure and component of SPR were determined by a JSM-6360LV scanning electronic microscope (SEM) coupled with a Genesis 60S energy dispersive X-ray analyzer (EDXA). The infrared spectrum of sample was performed by the KBr method using an AVATAR360 Fourier transform infrared spectrometer.
3 Results and discussion
3.1 Characteristics of sugarcane pulp residue
The surface morphology of SPR was observed by using a SEM (Fig.1). The original SPR is of a porous and rough structure in possession of large surface area, which is favorable for chromium ion adsorption. However, the SPR after adsorbing Cr(Ⅲ) and Cr(Ⅵ) shows plenty of newly shiny particles over the surface of adsorbent, which is probably contributed to ions adsorption on the SPR. The EDAX spectrum further reveals that the SPR consists of 65.80% of carbon, 32.94% of oxygen, 0.64% of silicon and 0.61% of potassium, respectively.

Fig.1 SEM images of SPR: (a) Original; (b) Cr(Ⅲ)-loaded SPR; (c) Cr(Ⅵ)-loaded SPR
The infrared spectrum analysis was used to determine the vibrational frequency changes in the functional groups of the SPR (Fig.2). The spectra of SPR were measured within the wave number range of 400-4 000 cm-1. For original SPR, the broad stretching intense peaks at 3 383.2, 2 918.1, 1 730.5, and 1 054.2 cm-1 represent hydroxyl (—OH), alkyl (C—H), carboxyl (C=O) and ether (C—O), respectively. When SPR is loaded with Cr(Ⅵ), the band peaks of carboxyl (C=O), ether (C—O) and hydroxyl (—OH) groups shift to 1 731.5, 1 055.4, and 3 407.2 cm-1, respectively. However, only a peak shift of hydroxyl (—OH) group is observed in the Cr(Ⅲ)-loaded SPR. The results indicate that different groups are involved in Cr(Ⅲ) and Cr(Ⅵ) adsorption, leading to different removal behaviors between Cr(Ⅲ) and Cr(Ⅵ).
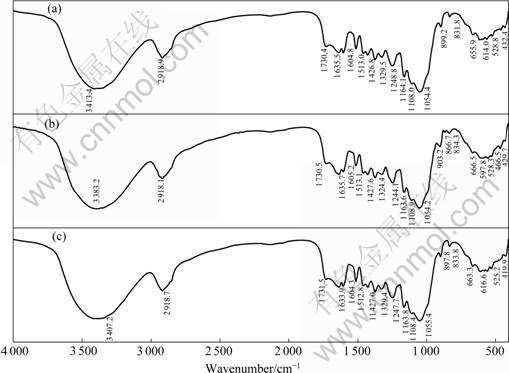
Fig.2 Infrared spectra of SPR: (a) Cr(III)-loaded SPR; (b) Original; (c) Cr(VI)-loaded SPR
3.2 Effect of pH value on Cr(Ⅲ) and Cr(Ⅵ) removal
The effect of pH values on chromium ion removal was investigated over a pH range varying from 2 to 8 in the present study. As shown in Fig.3, Cr(Ⅲ) and Cr(Ⅵ) removal by SPR is highly pH-dependent. The removal percentage of Cr(Ⅵ) decreases sharply in the pH value range from 2.0 to 5.5 and then slightly increases with increasing pH value. At pH=2.0, more than 80% of Cr(Ⅵ) in solution is removed by SPR. The corresponding value is 29.1% at pH=5.5. The effect of pH values on Cr(VI) adsorption on SPR may be attributed to both nature of SPR and the adsorbed Cr(Ⅵ) species since there is a competition between Cr(Ⅵ) and proton for the binding sites on SPR surface. The most predominant forms of Cr(Ⅵ) in aqueous system are acidic chromates(HCrO4-), chromates(CrO42-), and dichromates(Cr2O72-). From the stability diagram for the Cr(Ⅵ)-H2O system, it is evident that acidic chromate ions are the dominant species at low pH value. As pH increases, the proportion of acidic chromate ions in solution decreases, which may be caused by a decrease in net positive centers on the SPR surface. Consequently, the weakness of electrostatic forces between the adsorbed ions and adsorbent leads to a decrease in the sorption capacity.
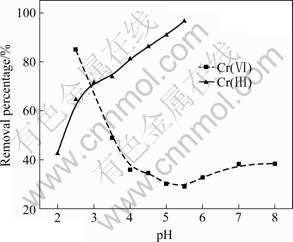
Fig.3 Effect of pH value on chromium removal percentage by SPR (Chromium concentration: 50 mg/L of Cr(Ⅵ) and 100 mg/L of Cr(Ⅲ); SPR dosage: 0.5 g; Shaking time: 24 h; Temperature: (28±1)℃)
Unlike Cr(VI), the removal percentage of Cr(Ⅲ) increases with increasing pH values (Fig.3). At pH=5.0,more than 90% of Cr(Ⅲ) in solution is removed and the corresponding removal percentage of Cr(Ⅲ) at pH=2.0 is only 42.5%. Such sorption trend is attributed to the competition between Cr(Ⅲ) and proton for the binding sites on SPR surface. At low pH value, an excess proton can compete with Cr(Ⅲ), resulting in a low percentage of Cr(Ⅲ) adsorption. Increasing the pH value can decrease the amount of the competitive H+ ion in the system. Therefore, the metal ion loading becomes more probable[17]. However, precipitate could not be excluded at a higher pH value. To avoid precipitation of Cr(Ⅲ), all the experiments on Cr(Ⅲ) adsorption are conducted at a maximum pH value of 5.0.
3.3 Effect of SPR dosage on Cr(Ⅲ) and Cr(Ⅵ) removal
The effect of SPR dosage on Cr(Ⅲ) and Cr(Ⅵ) removal was investigated by changing the adsorbent dose from 0.2 to 1.2 g with the initial Cr(Ⅲ) concentration of 100 mg/L and the initial Cr(Ⅵ) concentration of 50 mg/L. The results are presented in Fig.4. As can be seen, the removal percentage of chromium increases with increasing SPR dosage and then reaches the maximum with more than 90% of Cr(Ⅲ) and more than 60% of Cr(Ⅵ) in solution being removed at 0.8 g of SPR. At a low SPR dosage, e.g. 0.2 g, the available adsorption sites are quite insufficient compared with those of the large amount of chromium ions in solution, resulting in low removal percentage. At a higher SPR dosage, e.g. 0.8 g, the adsorption sites are sufficient, and a further increase in SPR dosage does not lead to a significant increase in the chromium removal percentage from aqueous solution. Moreover, at a higher SPR dosage, the effective contact surface area of the adsorbent declines, resulting in a decrease of unit adsorption capacity.
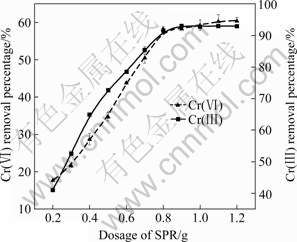
Fig.4 Effect of SPR dosage on chromium removal percentage (Chromium concentration: 50 mg/L of Cr(Ⅵ) and 100 mg/L of Cr(Ⅲ); Shaking time: 24 h; Temperature: (28±1) ℃)
3.4 Effect of SPR particle size on Cr(Ⅲ) and Cr(Ⅵ) removal
The influence of SPR particle size on chromium removal is shown in Fig.5. As the SPR particle size decreases from 0.450 to 0.075 mm, the removal percentage increases from 23 % to 43% for Cr(Ⅵ) and from 52% to 84% for Cr(Ⅲ), respectively. The increased removal percentage of chromium caused by the decrease of SPR particle size is due to the large adsorption surface for the small particle size. Generally, at the same dosage of SPR, the smaller particle size can supply more sites for ion adsorption, resulting in higher removal percentage.

Fig.5 Effect of SPR particle size on chromium removal percentage (Chromium concentration: 50 mg/L of Cr(Ⅵ) and 100 mg/L of Cr(Ⅲ); SPR dosage: 0.5 g ; Shaking time: 24 h; Temperature: (28±1)℃)
3.5 Effect of temperature on Cr(Ⅲ) and Cr(Ⅵ) removal
The removal percentages of Cr(Ⅲ) and Cr(Ⅵ) by SPR were investigated as functions of temperatures (288, 296, 303, 313, 323, and 333 K) in batch experiments when the initial concentrations of Cr(Ⅲ) and Cr(Ⅵ) were 100 and 50 mg/L, respectively. There is an increase in the removal percentage of Cr(Ⅲ) and Cr(Ⅵ) with rising temperature. For instance, Cr(Ⅲ) removal percentage increases from 65% at 288 K to 85% at 333 K. Correspondingly, Cr(Ⅵ) removal percentage increases from 26% at 288 K to 44% at 333 K.
The uniformity or heterogeneity of the surface sites of SPR was deduced from the isosteric heat of adsorption as a function of adsorption density using the Clausius- Clapeyron equation[15]. The isosteric heat of adsorption can be calculated from adsorption isotherms at two different temperatures:
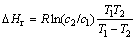 (1)
(1)
where ?Hr is the isosteric heat of adsorption in kJ/mol at a given adsorption density, R is the gas constant, and c1 and c2 are the equilibrium concentrations of the ion at temperatures T1 and T2, respectively. If the isosteric heat of adsorption is independent of the adsorption density, then the surface is homogenous. However, if it decreases with increasing adsorption density, then the surface is heterogenous[18]. In this work, an increase in the adsorption percentage of Cr(Ⅲ) and Cr(Ⅵ) with a rise in temperature and the variation in the isosteric heat of adsorption support the heterogenous nature of SPR (Table 1).
Table 1 Isosteric heat of adsorption for Cr(VI) and Cr(III)

3.6 Effect of initial chromium concentration on Cr(Ⅲ) and Cr(Ⅵ) removal
The adsorption amounts and removal percentages of Cr(Ⅲ) and Cr(Ⅵ) with varying chromium concentration are presented in Fig.6. The adsorption amount of Cr(Ⅲ) on SPR rapidly increases from 1.13 to 2.80 mg/g when the initial Cr(Ⅲ) concentration varies from 40 to 200 mg/L. Thereafter, Cr(Ⅲ) adsorption amount slightly increases. Conversely, the removal percentage of Cr(Ⅲ) decreases with increasing Cr(Ⅲ) concentration, which is contributed to the limited binding sites of SPR. The similar adsorption trends are also found for Cr(Ⅵ) adsorption. However, the adsorption amount and removal percentage of Cr(Ⅵ) are distinctly lower than those of Cr(Ⅲ).
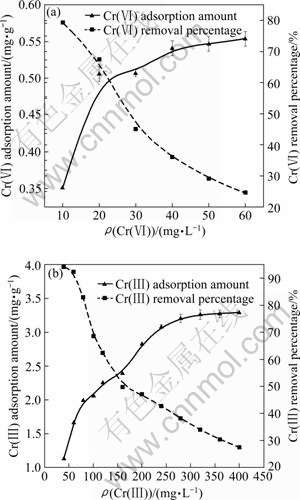
Fig.6 Effect of initial ion concentration on the adsorption amount and removal percentage of Cr(Ⅵ) (a) and Cr(Ⅲ) (b) removal (SPR dosage: 0.5 g; Shaking time: 24 h; Temperature: (28±1) ℃)
3.7 Adsorption kinetics of Cr(Ⅲ) and Cr(Ⅵ)
The removal percentages of Cr(Ⅲ) and Cr(Ⅵ) with adsorption time are shown in Fig.7. The removal percentages of Cr(Ⅲ) and Cr(Ⅵ) increase rapidly within the first 24 h, and more than 35% of Cr(Ⅵ) and more than 55% of Cr(Ⅲ) are removed. Thereafter, the removal percentages of Cr(Ⅲ) and Cr(Ⅵ) increase slowly. The results indicate that Cr(Ⅲ) and Cr(Ⅵ) adsorption on SPR can reach equilibrium at 24 h.
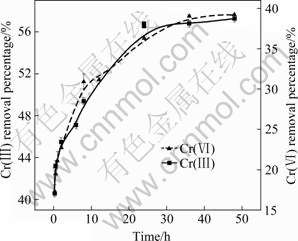
Fig.7 Chromium removal percentage by SPR with adsorption time (Chromium concentration: 50 mg/L of Cr(Ⅵ) and 200 mg/L of Cr(Ⅲ); SPR dosage: 0.5 g; Temperature: (28±1) ℃)
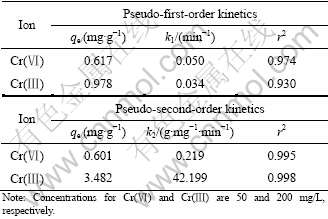
In order to investigate the mechanism of Cr(Ⅲ) and Cr(Ⅵ) adsorption on SPR and to examine the rate-controlling step of the adsorption process, the kinetic data obtained were analyzed by using the pseudo-first-order kinetics model and pseudo-second- order kinetics model[19-20]. Table 2 shows the predicted parameters for the above kinetic models. The correlation coefficient (r2) of pseudo-first-order model for Cr(Ⅲ) adsorption on SPR is 0.930 and the equilibrium adsorption capacity (qe) is 0.978 mg/g. The predicted adsorption capacity of Cr(Ⅲ) is much lower than the actual amount of equilibrium adsorption (3.480 mg/g). Therefore, the pseudo-first-order is an undesired model for Cr(Ⅲ) adsorption on SPR. The kinetic adsorption data of Cr(Ⅲ) are further fitted by the pseudo-second-order kinetic model.
The results show that the pseudo-second-order kinetic model is better than the pseudo-first-order kinetic model because the former model reveals a higher correlation coefficient (r2=0.998). Furthermore, the calculated equilibrium adsorption capacity (qe) of Cr(Ⅲ) is 3.482 mg/g, which is closer to the actual amount of adsorption equilibrium (3.480 mg/g). The results indicate that the pseudo-second-order kinetic model is more suitable for describing Cr(Ⅲ) adsorption kinetics. The pseudo-second-order model is based on the assumption that the rate-limiting step is a chemical sorption involving valance force through sharing or exchange of electrons between adsorbent and adsorbate[19, 21-22]. Successful fitting of this model suggests that chemical sorption of Cr(Ⅲ) is the rate-controlling step[19].
As shown in Table 2, the kinetic adsorption data of Cr(Ⅵ) are also better fitted with the pseudo-second-order kinetic model than with the pseudo-first-order kinetic model since the former kinetic model has higher correlation coefficient.
Table 2 Predicted kinetic parameters for chromium adsorption on SPR
3.8 Adsorption isotherms of Cr(Ⅲ) and Cr(Ⅵ)
The Langmuir isotherm is a commonly used adsorption isotherm for assessing the potential use of an adsorbent[22]. The Langmuir isotherm model assumes uniform energies of adsorption on the surface with no transmigration of adsorbate in the plane of the surface. The linearized isotherms with respect to the Langmuir model are depicted in Fig.8. In this work, the Langmuir isotherm provides an excellent fit to the equilibrium sorption data of Cr(Ⅲ) and Cr(Ⅵ), giving correlation coefficients of 0.992 and 0.999, respectively. The maximum adsorption capacity (qm) and adsorption equilibrium constant (k) for Cr(Ⅵ) are 0.567 mg/g and 0.787 L/mg, respectively. The corresponding qm and k values for Cr (Ⅲ) are 3.446 mg/g and 0.062 5 L/mg.
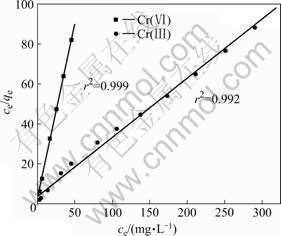
Fig.8 Langmuir isotherms for adsorption of Cr(VI) and Cr(III) on SPR
The shape of the Langmuir isotherm was investigated by the dimensionless constant separation term (RL) to determine whether the chromium adsorption by SPR is a high-affinity adsorption. RL is calculated as follows:
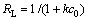 (2)
(2)
where c0 is the initial chromium concentration, μmol/L; k is the adsorption equilibrium constant related to bonding energy, L/mg. RL can be calculated from the slopes of the Langmuir constants. RL indicates the shape of the adsorption isotherm and 0<RL<1 corresponds to high-affinity adsorption. All calculated RL values indicate that chromium adsorption by using SPR is high-affinity adsorption regardless of the initial concentration tested here. For example, at the initial Cr(Ⅵ) concentration of 10 mg/L, RL equals 0.112 8. At the initial Cr(Ⅲ) concentration of 40 mg/L, RL equals 0.285 7. The low reversibility found here suggests that the adsorption is the high-affinity type and chemical adsorption may govern the system[23].
4 Conclusions
(1) Cr(Ⅵ) and Cr(Ⅲ) are removed by SPR in aqueous solution due to the strong adsorption on the active sites of SPR. However, SPR has higher adsorption capacity for Cr(Ⅲ) than that for Cr(Ⅵ).
(2) The adsorption kinetics of Cr(Ⅵ) and Cr(Ⅲ) well fit with pseudo-second-order model. Langmuir adsorption isotherm can describe chromium ions adsorption phenomena. Moreover, the adsorption of Cr(Ⅵ) and Cr(Ⅲ) on SPR is chemical adsorption process and the high-affinity type.
(3) SPR has more potential application in Cr(III)-containing wastewater treatment than that in Cr(Ⅵ)-containing wastewater treatment.
References
[1] SHANKER A K, CERVANTES C, LOZA-TAVERA H, AVUDAINAYAGAM S. Chromium toxicity in plants [J]. Environment International, 2005, 31(5): 739-753.
[2] MEGHARAJ M, AVUDAINAYAGAM S, NAIDU R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste [J]. Current Microbiology, 2003, 47(1): 51-54.
[3] GODE F, ATALAY E D, PEHLIVAN E. Removal of Cr(VI) from aqueous solutions using modified red pine sawdust [J]. Journal of Hazardous Materials, 2008, 152(3): 1201-1207.
[4] KOMGOLD E, BELAYEV N, ARONOY L. Removal of chromates from drinking water by anion exchangers [J]. Separation and Purification Technology, 2003, 33(2): 179-187.
[5] CHEN Yun-nen, CHAI Li-yuan, SHU Yu-de. Arsenic(Ⅴ) removal from drinking water by bone char [J]. Journal of Central South University: Science and Technology, 2008, 39(2): 279-283. (in Chinese)
[6] CHAI Li-yuan, CHEN Yun-nen, SHU Yu-de, CHANG Hao, LI Qing-zhu. Adsorption and removal of cadmium (Ⅱ) from aqueous solutions by bio-formulation [J]. Trans Nonferrous Met Soc China, 2007, 17(5): 1057-1062.
[7] MOHANTY K, JHA M, MEIKAP B C, BISWAS M N. Removal of chromium(Ⅵ) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride [J]. Chemical Engineering Science, 2005, 60(11): 3049-3059.
[8] LAZARIDIS N K, ASOUHIDOU D D. Kinetics of sorptive removal of chromium(Ⅵ) from aqueous solutions by calcined Mg-Al-CO3 hydrotalcite [J]. Water Research, 2003, 37(12): 2875-2882.
[9] TAN W T, OOI S T, LEE C K. Removal of chromium(Ⅵ) from solutions by coconut husk and palm pressed fibres [J]. Environmental Technology, 1993, 14(3): 277-282.
[10] YU L J, SHUKLA S S, DORRIS K L, SHUKLA A, MARGRAVE J L. Adsorption of chromium from aqueous solutions by maple sawdust [J]. Journal of Hazardous Materials, 2003, 100(1/3): 53-63.
[11] NAMASIVAYAM C, YAMUNA R T. Adsorption of chromium(Ⅵ) by a low-cost adsorbent [J]. Chemosphere, 1995, 30(3): 561-578.
[12] EROMOSELE I C, BAYERO S S. Adsorption of chromium and zinc ions from aqueous solution by celluosic graft copolymers [J]. Bioresource Technology, 2000, 71(3): 279-281.
[13] ROJAS G, SILVA J, FLORES J A, RODRIGUEZ A, LY M, MALDONADO H. Adsorption of chromium onto cross-linked chitosan [J]. Separation and Purification Technology, 2004, 44(1): 31-36.
[14] WU Yun, ZHANG Shu-zhen, GUO Xue-yan, HUANG Hong-lin. Adsorption of chromium(Ⅲ) on lignin [J]. Bioresource Technology, 2008, 99(16): 7709-7715.
[15] PRADHAN J, DAS S N, THAKUR R S. Adsorption of hexavalent chromium from aqueous solutions by using activated red mud [J]. Journal of Colloid and Interface Science, 1999, 217(1): 137-141.
[16] GUPTA V K, JAIN C K, ALI I, SHARMA M, SAINI V K. Removal of cadmium and nickel from wastewater using bagassess fly ash—A sugar industry waste [J]. Water Research, 2003, 37(16): 4038-4044.
[17] SHUKLA A, ZHANG Y H, DUBEY P, MARGRAVE J L, SHUKLA S S. The role of sawdust in the removal of unwanted materials from water [J]. Journal of Hazardous Materials, 2002, 95(1/2): 137-152.
[18] PARIDA K M, MALLICK S, DASH S S. Studies on manganese nodule leached residues. 2. Adsorption of aqueous phosphate on manganese nodule leached residues [J]. Journal of Colloid and Interface Science, 2005, 290(1): 22-27.
[19] HAMEED B H, AHMAD A A, AZIZ N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash [J]. Chemical Engineering, 2007, 133(1/3): 195-203.
[20] MOHAN D, SINGH K P, SINGH V P. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth [J]. Journal of Hazardous Materials, 2006, 135(1/3): 280-295.
[21] IBRAHIM K, MEHMET U, HAMDI K, ALI C. Adsorption of Cd(Ⅱ) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation [J]. Bioresource Technology, 2008, 99(3): 492-501.
[22] HO Y S. Selection of optimum sorption isotherm [J]. Carbon, 2004, 42(10): 2115-2116.
[23] ALTUNDOGAN H S, ALTUNDOGAN S, TUMEN F, BILDIK M. Arsenic removal from aqueous solutions by adsorption on red mud [J]. Waste Management, 2000, 20(8): 761-767.
Foundation item: Projects(2006AA06Z374, 2007AA021304) supported by the National High-Tech Research and Development Program of China; Project(2008SK2007) supported by the Key Program of Science and Technology of Hunan Province, China
Received date: 2008-12-02; Accepted date: 2008-12-20
Corresponding author: CHAI Li-yuan, Professor, PhD; Tel: +86-731-8836921; E-mail: lychai@mail.csu.edu.cn
(Edited by CHEN Wei-ping)