J. Cent. South Univ. Technol. (2008) 15(s1): 115-117
DOI: 10.1007/s11771-008-327-x
Mechanism of sulfide effect on viscosity of HPAM polymer solution
KANG Wan-li(康万利)1, 2, ZHOU Yang(周 阳)2, WANG Zhi-wei(王志伟)2,
MENG Ling-wei(孟令伟)2, LIU Shu-ren(刘述忍)2, BAI Bao-jun(白宝君)3
(1. College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China;
2. Enhanced Oil Recovery Center, China University of Petroleum, Qingdao 266555, China;
3. Missouri University of Science and Technology, Rolla, MO 65401, USA)
Abstract: The effect of sulfide on HPAM solution viscosity was studied using BROOKFIELD DV-II viscometer, and the interaction mechanism was discussed. The HPAM solution viscosity was investigated through fully reducing sulfide by the addition of hydrogen peroxide oxidation, and the mechanism of increasing polymer viscosity was investigated. The experimental results also show that there is a critical concentration of 15 mg/L. Below it, the loss rate of HPAM solution viscosity increases more rapidly, but becomes slowly above the critical concentration. A theoretical guidance for oilfields to prepare polymer solution using sewage-water by eliminating sulfide, and it is also importance to prepare polymer solution using sewage-water and save fresh water.
Key words: sulfide; HPAM solution; mechanism of viscosity reduction; viscosity loss rate
1 Introduction
In recent years, with the increase of the polymer flooding application, the strain of fresh water source for preparing polymer solution becomes more and more serious. On the other hand, the produced water from oilfields can not be reinjected in the same amount. This not only wastes the resource, but also increases the oilfield operation cost. The factors affecting polymer solution viscosity are involved in chemical factors, mechanical factors and biological factors. Salt, temperature and oxygen are the main chemical factors. Especially in produced water, plenty of salt, iron and sulfide can affect polymer solution viscosity[1]. The mechanisms of salinity on HPAM solution viscosity were studied deeply by KANG et al[2]. The effects of oxygen and temperature were discussed by ZHU et al[3-5]. In addition, the iron and sulfide in produced water also influence polymer viscosity[6-7]. Oxygen aeration is often used to treat oilfield produced water[8-10]. However, there is little report about the systematic research on the effect of separate sulfide in oilfield produced water on polymer solution viscosity. In this paper, the interaction mechanism of sulfide in synthesized produced water on polymer solution viscosity was reported.
2 Experimental
2.1 Materials and instruments
1) Materials: HPAM with relative molecular mass of 2 200×104, Na2S·9H2O, deionized water, hydrogen peroxide (produced by Yantai Sanhe Chemical Limited Corporation) with mass fraction of 30%.
2) Instrument: Brookfield DV-II Viscometer
2.2 Experimental scheme
1) Sulfide content simulation
NaCl solution was prepared with the concentration of 2.125 6, 3.105 7 and 6.818 8 g/L, respectively. The concentration of S2- under different concentration brine was simulated using saline solutions with different concentrations prepared with Na2S. The polymer solution concentration was 1.300 g/L, and the experimental temperature was 45 ℃.
2) Desulfurizing through hydrogen peroxide oxidation
The concentration of S2- was 15, 7 and 45 mg/L, respectively, corresponding to the concentrations of NaCl solutions of 2.125 6, 3.105 7 and 6.818 8 g/L. H2O2 solutions of different concentration were added into the simulated solution. The reaction temperature was 60 ℃, and the reaction time was 20 min, and then the simulated solution was cooled to room temperature. The polymer solution was prepared at the concentration of 1.300 g/L and the volume of 100 mL, and the measuring temperature was 45 ℃.
3 Results and discussion
3.1 Effect of sulfide content on HPAM solution viscosity
Polymer solution viscosity changes with the increase of the concentration of S2- ion as shown in Fig.1. The polymer solution viscosity obviously reduces with the increase of S2- concentration for different concentration brine, and there is a critical concentration of 15 mg/L, below which polymer solution viscosity reduces fast as the S2- concentration increases, while above which, polymer solution viscosity reduces slowly with the increase of the S2- concentration further.
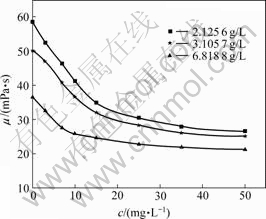
Fig.1 Effect of S2- concentration on HPAM viscosity
3.2 Effect of desulfurizion by hydrogen peroxide oxidation on HPAM viscosity
Fig.2 shows the effect of H2O2 concentration on polymer solution viscosity for each constant S2- concentration. Polymer solution viscosity increases with

Fig.2 Relationship between HPAM viscosity and H2O2 concentration at fixed S2- concentration
the increase of H2O2 concentration. The maximum HPAM solutions viscosity containing sulfide content of 15, 7 and 45 mg/L, respectively, appears when H2O2 concentrations are about 25, 10 and 45 mg/L, respectively.
The mechanism of desulfurizing by hydrogen peroxide oxidation is that O2 is separated from the hydrogen peroxide solution heated which has strong ability of oxygen-quality. Then the sulfide in the waste water is oxided to elemental sulfur by O2, which will be removed through the filter. The reaction formula can be expressed as follows.
2H2O2=2H2O+O2↑
2H2O+2S2-+O2=2S↓+4OH-
Fig.2 indicates there is a maximum viscosity with the increase of H2O2 for each concentration sufide solution. The required H2O2 amount to reach the maximum polymer viscosity is dependent on sufide concentration. When the concentration of H2O2 reaches the maximum point, the sulfide is just oxided, or slightly more than this concentration. Polymer solution viscosity increases fastly with the increase of H2O2 concentration for the solution with sulfide of 7 and 15 mg/L at the beginning. The highest polymer solution viscosity appears when the sulfide in simulation is just oxided by H2O2. Then, the viscosity of the polymer solution declines in some degree with the increase of H2O2 concentration further. But the polymer solution viscosity slightly increases with H2O2 concentration for the solution with sulfide of 45 mg/L at the beginning. Polymer solution viscosity recovers quickly with the increase of H2O2 concentration after H2O2 concentration reaches about 30 mg/L.
Comparing Figs.1 and 2, it indicates that polymer solution viscosity increases rapidly because sulfide content in polymer solution has great impact on polymer solution viscosity when the sulfide concentrations in the simulate solution are 7 and 15 mg/L. A small amount of sulfide reduction plays a significant role in improving polymer solution viscosity in this range. But a small amount of sulfide reduction has a little impact on the recovery of polymer solution viscosity because the sulfide with concentration 45 mg/L reaches high level. Therefore, polymer solution viscosity recovers slowly. With H2O2 concentration further increases, the content of sulfide decreases to the range in which the content of sulfide has larger impact on polymer solution viscosity. Therefore, polymer solution viscosity recovery soon. With the further increase of hydrogen peroxide concentration, the polymer solution viscosity reduces because the oxygen separates from the hydrogen peroxide solution which dissolves in polymer solution and plays a significant role in the degradation of polymer solution.
4 Mechanism of sulfide viscosity of polymer solution
The degradation reaction of HPAM chain belongs to free radical reaction. The reason for the degradation of HPAM chain is that the weak points in the polymer chain are broken. The process of free radical reactions can be expressed as follows[11].
P-H (polymer molecules) + O2→P ? + HOO ? (1)
where P ? is the polymer chain containing free radicals. The polymer radical P ? will react with oxygen rapidly if there are reducing substances in polymer solution such as sulfide. The following peroxide radicals can be produced.
P ? + O2 POO ? (2)
POO ? (2)
The following chain propagation reaction will be caused by these free peroxide radicals, and hydrogen peroxide polymer POOH is produced.
POO ? + PH→POOH + P ? (3)
More free radicals can be produced through the decomposition of polymer hydrogen peroxide. These series of reactions will not end up until oxygen is consumed completely. The free radical reaction that chemical activity is very lively and will lead to polymer matrix rupture, which results in serious loss of polymer solution viscosity.
The research results show that the reduce of the substances in polymer solution will speed up these reactions and the loss of polymer solution viscosity. The degradation reaction of HPAM can be considered as three steps as follows. First, free radical is produced by the decomposition of impurities of peroxide contained in polymer products, which leads to a reaction chain (Eqns.(1)-(3)). Second, these reactions are accelerated significantly when the temperature is elevated or there are impurities of reduction in polymer solution. Third, polymer main chain breaks, which is initiated by the free radicals of polymer chain and results in significant reduction of polymer solution viscosity.
The interaction mechanism of sulfide on HPAM solution viscosity reduction is that divalent sulfur ion reducing substances in HPAM solution accelerates the radical reaction degradation in the solution. Then, the polymer main chain breaks and accelerates polymer degradation greatly, which is initiated by free radicals of polymer chain. Therefore, polymer solution viscosity reduces rapidly and the retention rate of viscosity is very low.
5 Conclusions
1) Sulfide significantly reduces HPAM solution viscosity. The main reason is that the divalent sulfur ion can accelerate polymer free radicals degradation. There is a critical concentration of 15 mg/L for the tested solutions, below which, HPAM solution viscosity reduces more fast while above it HPAM solution viscosity reduces slowly.
2) Hydrogen peroxide can be used to efficiently desulfurize the HPAM solution viscosity. The mechanism of eliminating sulfide by the hydrogen peroxide oxidation is obtained.
References
[1] FAN Ping, WANG Xue-jun, WANG Xiao-yu, HU Jun-qing. Research on demineralization method of wastewater from oilfield[J]. Environmental Science and Management, 2007, 32(1): 90-93. (in Chinese)
[2] KANG Wan-li, MENG Ling-wei, NIU Jing-gang, XU Dian-ping. Mechanism of the effect of salinity on HPAM solution viscosity[J]. Polymer Materials Science and Engineering, 2006, 22(5): 175-177. (in Chinese)
[3] ZHU Lin-yong, CHANG Zhi-ying, LI Miao-zhen, WANG Er-jian. Oxidative degradation of partially hydrolyzed polyacrylamide in aqueous solutionⅠ influence of temperature[J]. Polymer Materials Science and Engineering, 2000, 16(1): 113-116. (in Chinese)
[4] ZHU Lin-yong, CHANG Zhi-ying, LI Ming-yu, WANG Er-jian. Oxidative degradation of partially hydrolyzed polyacrylamide in aqueous solution Ⅱ. Influence of organic impurities[J]. Polymer Materials Science and Engineering, 2000, 16(2): 112-114. (in Chinese)
[5] ZHU Lin-yong, CHANG Zhi-ying, MA Chang-qi, LI Miao-zhen, WANG Er-jian. Oxidative degradation of partially hydrolyzed polyacrylamide in aqueous solutionⅢ stability at high temperature[J]. Polymer Materials Science and Engineering, 2002, 18(2): 93-96. (in Chinese)
[6] YANG Huai-jun, LUO Ping-ya. Factors for and control of polymer degradation in recycled produced water solutions[J]. Oil Chemistry, 2005, 22(2): 158-162. (in Chinese)
[7] LUO Yi-jing, ZHANG Zhong-zhi, ZHAO Shu-ying, SONG Shao-fu, HUANG Jie. Progressive research of treatment for sewage produced from polymer flooding displacement[J]. Journal of Petrochemical Universities, 2003, 16(1): 9-13. (in Chinese)
[8] YAO Lan, SUN Chun-hong, SU Yan-chang. Method of displacement with polymer solution diluted with produced water[J]. Petroleum Geology and Oilfield Development in Daqing, 2005, 24(3): 79-80. (in Chinese)
[9] WANG Bao-jiang, LI Yan-xing, YAO Lan, SUN Chun-hong. Test of using fresh water to formulate sewage dilution polymer solution [J]. Petroleum Geology and Oilfield Development in Daqing, 2001, 20(2): 86-91. (in Chinese)
[10] ZHANG Ke, JIANG Wei-dong, LU Xiang-guo, ZHAO Jin-yi, LI Bai-guang. A laboratory study on impact of dissolved oxygen to viscosity of polymer solutions prepared in oilfield produced water[J]. Oilfield Chemistry, 2006, 23(3): 239-242. (in Chinese)
[11] FU Mei-long, ZHOU Ke-hou ZHAO Lin. Effect of dissolved oxygen on polymer stability[J]. Journal of Southwest Petroleum Institute, 1999, 21(1): 71-73. (in Chinese)
(Edited by LI Yan-hong)
Foundation item: Project(200873181) supported by NSFC; Project(2007AA06Z214) supported by the High-tech Research and Development Program of China; Project(20070704) supported by Taishan Scholars Construction Engineering
Received date: 2008-06-25; Accepted date: 2008-08-05
Corresponding author: KANG Wan-li, Professor, PhD; Tel: +86-13589332193; E-mail: Kangwanli@126.com