


Trans. Nonferrous Met. Soc. China 22(2012) 943-948
A novel and facile wet-chemical method for synthesis of silver microwires
ZHAI Ai-xia1, CAI Xiong-hui2, JIANG Xiao-ye2, FAN Guo-zhi1
1. School of Chemical and Environmental Engineering, Wuhan Polytechnic University, Wuhan 430023, China;
2. School of Biologic and Pharmaceutical Engineering, Wuhan Polytechnic University, Wuhan 430023, China
Received 9 August 2011; accepted 14 December 2011
Abstract: A novel and facile wet-chemical method for synthesis of silver microwires was developed. The well-defined particles were prepared by adding an iron (II) sulfate heptahydrate solution into a silver nitrate solution containing citric acid drop by drop at 50 ℃. The resulting products were characterized by scanning electron microscopy and X-ray diffraction. It was found that the particles consisted of numerous silver microwires. The reaction temperature greatly affected the morphologies of the as-prepared particles. Both of the mean length and width of the silver microwires increased with the decrease of the concentration of silver nitrate. And the lower concentration was unfavorable for the formation of more silver microwires. Similar findings were also observed when the concentration of iron (II) sulfate was decreased. The amount of citric acid also greatly affected the shape of the as-prepared particles. It was concluded that citric acid was the key role in the formation of silver microwires via the Oswald ripening mechanism.
Key words: silver microwires; iron (II) sulfate heptahydrate; citric acid; wet-chemical method
1 Introduction
Systematically manipulating the one-dimensional (1D) metal nano/micro structures has gained increasing interest because of their unique electrical, optical, thermal, and mechanical properties and the potential applications in electronics, photonics, (bio)chemical sensing and imaging, catalysis, and so on [1-3]. This is particularly true for 1D silver nano/microwires. They have shown potential in plasmonic waveguiding [4-6], surface-enhanced Raman spectroscopy [7-9], catalysis [10], photoluminescence [11], electrical and thermal conductivity [12], biological and chemical sensing [13], and the templating synthesis of other 1D metals [14-16]. Consequently, considerable effort has been devoted to the synthesis of silver nano/microwires. The wet-chemical methods are severely preferred for the advantage of controlling the particle morphology and size. Currently, the most commonly used wet-chemical methods for the preparation of silver nano/microwires involve “hard templates,” such as porous anodic alumina membranes [17,18], polycarbonate [19], and carbon nanotubes [20], “soft templates,” including DNA chains [21-23], double-hydrophilic block copolymer [24], poly(methacrylic acid) [25], polyacrylic acid [26], L-cysteine [27], and hydrothermal or solvothermal synthesis, in which ethylene glycol [28-32], ethanol [33], citrate ion [34-36], tartaric ion [37], methanol [38] N,N-dimethylformamide [39], and methenamine [40] were used as reductants of silver ions. Clearly, the synthesis template often needs to be removed to obtain the pure silver nano/microwires in the template-directed synthesis. And the hydrothermal or solvothermal synthesis processes are often carried out above 100 ?C. Thus, the exploration of novel wet-chemical methods for the synthesis of silver nano/microwires is still needed. Here, a novel and facile wet-chemical method for the synthesis of silver microwires is presented. The defined silver microcrystals are prepared by adding an iron (II) sulfate heptahydrate solution into a silver nitrate solution containing citric acid drop by drop at 50 ℃.
2 Experimental
2.1 Materials
Silver nitrate (AgNO3, analytical reagent, Shanghai Chemical Reagent Co., Ltd.), iron (II) sulfate heptahydrate (FeSO4·7H2O, analytical reagent, Sinopharm Chemical Reagent Co., Ltd.), citric acid (C6H8O7·H2O, analytical reagent, Shanghai Chemical Reagent Co., Ltd.) and deionized water were used as raw materials without further purification.
2.2 Methods
In a typical procedure, the silver nitrate and the iron (II) sulfate heptahydrate solutions were prepared by dissolving 21 mg silver nitrate and 50 mg iron (II) sulfate heptahydrate in 10 mL deionized water respectively. The citric acid solution was also obtained by dissolving 19.2 mg citric acid in 3 mL deionized water. After the citric acid solution was mixed into the silver nitrate solution, the iron (II) sulfate solution was uniformly added into the mixed solution drop by drop at 50 ℃ in 30 min. The precipitates were separated from the solution and washed with deionized water 3-4 times and then with ethanol 2-3 times. After they had been desiccated in a vacuum desiccator at 50 ℃ for 30 min, the powders were obtained.
The powders were characterized by scanning electron microscopy (SEM, S-3000, Hitachi) and X-ray diffraction (XRD, X’Pert PRO, PANalytical B.V.).
3 Results and discussion
Typical SEM images of as-prepared powders are shown in Fig. 1. The powders consist of many microwires (Fig. 1(a)). And the mean length and width of them are about 10 μm and 0.2 μm, respectively. And there are also several agglomerated nanoparticles among the microwires (Fig. 1(b)).
The XRD pattern of the product is shown in Fig. 2. Significant diffraction peaks are located at 38.3°, 44.4°, 64.6°, and 77.6°. And they correspond respectively to the (111), (200), (220), and (311) plane diffraction peaks of the metallic silver cubic structure, indicating that the powders are composed of pure crystalline silver.
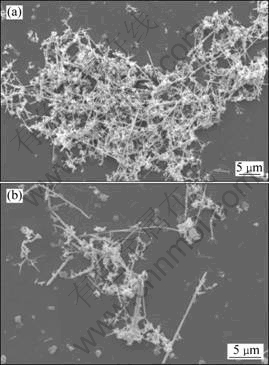
Fig. 1 Typical SEM images of silver microwires with low magnification (a) and high magnification (b)

Fig. 2 XRD pattern of as-prepared silver microwires
The reaction temperature was 50 ℃ according to the description of the synthesis above. The citrate ions, as reducing agents, are often used in a boiling solution [34-36]. And iron (II) sulfate heptahydrate is often considerably more capable than citrate ions reducing silver ions. Hence, the citric acid acted as an additive rather than a reductant in the proposed system. The reaction thus can be formulated as follows:

The formation of silver microcrystal commonly includes the production of nuclei and the growth of nuclei in aqueous solutions. The latter has a considerable effect on the final morphologies of the silver microcrystals, which is related to the reaction rate and the mediation of additives. Citric acid, an organic polybasic acid, with three carboxyls and one hydroxyl, shows typical chemical and physical characteristics of carboxylic acids in an aqueous solution. Citric acid acted as a complexant of silver ions and as a capping agent of silver nuclei when it was added to a silver nitrate solution. During the synthesis process, on one hand, the reduction rate decreased for the complexation of citrate ions ( dissociated from citric acid ) with silver ions. On the other hand, citrate ions could selectively adsorb onto certain crystal facets of the formed Ag nuclei. As documented in the literature, the plane (111) of silver possesses the lowest surface energy and can adsorb suitable additives, such as citrate ions [41,42]. This preferential adsorption of citrate ions onto the Ag (111) plane could promote crystal growth along other planes (such as the (110) plane) of the Ag nuclei. Both of them were favorable for the anisotropic growth of silver nuclei into 1D structures through the Ostwald ripening mechanism [43,44] in the presence of Fe (III), which was produced through the reduction reaction of silver ions. It is a well-established wet etchant for silver and other noble metals [45]. Its role in promoting the Ostwald ripening process requires further study. Thus, silver microwires were prepared.
3.1 Effect of reaction temperature
To investigate the effect of the reaction temperature on the morphologies of silver powders, the particles were prepared at 22 and 80 ℃ while other conditions were kept the same as the description in section 2.2. The resulting microwires were then compared with the particles obtained at 50 ℃. The results are shown in Fig. 3.
It is obvious that the reaction temperature has much impact on the morphologies of the resulting silver particles. At the reaction temperature of 22 ℃, the silver powders consist of a number of agglomerated silver nanoparticles, and few silver microwires are observed (Fig. 3(a)). When the reaction temperature is 50 ℃, more silver microwires are observed (Fig. 3(b)). However, when the reaction temperature is further increased to 80 ℃, few silver microwires are observed again, but their width is larger (Fig. 3(c)). The Oswald ripening mechanism is known to have a close relationship with the reaction temperature and time, which can be described by the equation (D-D0)=Kt1/n, where t is the time, K is a temperature-dependent material constant appropriate to the value of the exponent n, D and D0 are the average particle diameters at t and 0, respectively [46]. Referring to the equation, the reaction temperature has an effect on the morphology of the resulting silver particles. The relationship of the silver particle morphologies with the reaction temperature shown in Fig. 3 also supports the hypothesis that silver microwires were formed through the Oswald ripening mechanism mentioned above. In the present study, the dissolution rate of smaller nuclei at 22 ℃ was so slow that Oswald ripening almost did not occur. As a result, only agglomerated silver nanoparticles were obtained and silver microwires were not prepared. It is similar to the work of DU et al [33]. When the reaction temperature was 80 ℃, the reduction rate and the ripening rate of the silver ion also increased. However, the former may be increased much more, leading to the production of a large number of silver nuclei within a short time at the initial stage of the reaction. And most of the nuclei agglomerated instantly, which was not beneficial for the anisotropic growth of silver nuclei into 1D structures through the Oswald ripening mechanism.

Fig. 3 Typical SEM images of silver microwires obtained at reaction temperatures of 22 ℃ (a), 50 ℃ (b), and 80 ℃ (c)
3.2 Effect of concentration of silver nitrate
The effect of concentration of silver nitrate on the particle morphologies was also examined. Keeping the molar ratio of Ag+ to Fe2+ a constant, the silver particles were prepared at the 50% concentration of silver nitrate described in section 2.2. And other conditions were kept constants. The results are shown in Fig.4. The width and the length of the silver microwires prepared at the lower silver nitrate concentration (Fig. 4(b)) are larger than those prepared in section 2.2 (Fig. 4(a)). The average length and width of the microcrystals are 25 μm and 0.6 μm, respectively (Fig. 4(b)). But the yield of silver microwires is few. The reduction rate decreased when the concentration of silver nitrate was decreased to 1.05 g/L, resulting in fewer silver nuclei. But it was beneficial for the formation of the larger silver nuclei, that is, the D0 increased. According to the Oswald ripening equation, the D increases with the increase of D0. Hence, it is favorable for the formation of wider and longer silver microwires at the lower concentration of silver nitrate. However, the Ostwald ripening process was difficult to achieve because of the slow dissolution rate of the larger silver nuclei, which resulted in a lower yield of silver microwires.

Fig. 4 Typical SEM images of silver microwires obtained at concentrations of silver nitrate of 2.1 g/L (a) and 1.05 g/L (b)
3.3 Effect of concentration of iron (II) sulfate
To clarify the behavior of the growth of silver microcrystals at different concentrations of the reductant, systematic experiments were carried out at different concentrations of iron (II) sulfate. The silver particles were synthesized at the 50% concentration of iron (II) sulfate described in section 2.2, while other conditions remained unchanged. The results are shown in Fig. 5.
The morphologies of the prepared silver powders also changed when the concentration of iron (II) sulfate solution was decreased from 5.0 g/L to 2.5 g/L. When the concentration is 5.0 g/L, the powders consist of many silver wires (Fig. 5(a)). And when the concentration is 2.5 g/L, the powders mainly consist of agglomerated silver nano particles (Fig. 5(b)). The reduction rate of silver ion decreased with the decrease of the concentration of iron (II) sulfate solution. This is similar to the description in section 3.2, the concentration of silver nuclei decreased and larger silver nuclei were produced. It is not favorable for the formation of silver microwires via Oswald ripening. Thus, a large number of silver nuclei agglomerated.
3.4 Effect of amount of citric acid
As previously mentioned, citric acid acted as a complexant of the silver ions and as a capping agent of the silver nuclei during the synthesis of silver microwires. Silver particles were prepared at different amounts of citric acid to investigate the influence of citric acid on the formation of silver microwires. The results are shown in Fig. 6.
The powders consist of numerous silver microwires when the amount of citric acid is 38.4 mg (Fig. 6(a)). But when citric acid is 6.4 mg, most of the particles are silver nano crystals (Fig. 6(b)). In the described process, the citric acid acted as a capping agent of silver nuclei and a complexant of the silver ions. So, under the mediation of it, the anisotropic growth of the silver nuclei through the Ostwald ripening mechanism occurred and silver microwires were produced. When the amount of citric acid in the solution was 6.4 mg, the citrate ions were insufficient to cover all the certain crystal facet of the silver nuclei. And at the same time, the reaction rate was higher for the lower concentration of citrate ions. Both of them would affect the anisotropic growth of the silver nuclei through the Ostwald ripening mechanism. Consequently, fewer silver microwires were synthesized and more agglomerated silver nanoparticles were produced. Therefore, enough citric acid is necessary for the synthesis of many silver microwires in the present method. And it is concluded that citric acid played a key role in the formation of silver microwires.

Fig. 5 Typical SEM images of silver microwires obtained at concentrations of iron (II) sulfate solution of 5.0 g/L (a) and 2.5 g/L (b)

Fig. 6 Typical SEM images of silver microwires obtained at amount of citric acid of 38.4 mg (a) and 6.4 mg (b)
4 Conclusions
1) Silver microwires can be synthesized by adding iron (II) sulfate heptahydrate solution into silver nitrate solution containing citric acid drop by drop at 50 ℃.
2) The reaction temperature significantly affects the morphologies of as-prepared particles. Too high or too low temperature, it is not beneficial for the formation of silver microwires. Lower concentrations of silver nitrate and iron (II) sulfate are not favorable for the synthesis of numerous long and thin silver microwires.
3) Citric acid is the key role in the formation of silver microwires through the Oswald ripening mechanism, and an adequate amount of citric acid is necessary for the synthesis of silver microwires in this presented method.
References
[1] CHEN J, WILEY B J, XIA Y N. One-dimensional nanostructures of metals: Large-scale synthesis and some potential applications [J]. Langmuir, 2007, 23(8): 4120-4129.
Large-scale synthesis and some potential applications [J]. Langmuir, 2007, 23(8): 4120-4129.
[2] MURPHY C J, SAU T K, GOLE A M, ORENDORFF C J, GAO J X, GOU L F, HUNYADI S E, LI T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications [J]. J Phys Chem B, 2005, 109(29): 13857-13870.
[3] MURPHY C J, GOLE A M, HUNYADI S E, ORENDORFF C J. One-dimensional colloidal gold and silver nanostructures [J]. Inorg Chem, 2006, 45(19): 7544-7554.
[4] LI Z P, HAO F, HUANG Y Z, FANG Y R, NORDLANDER P, XU H X. Directional light emission from propagating surface plasmons of silver nanowires [J]. Nano Lett, 2009, 9(12): 4383-4386.
[5] FEDUTIK Y, TEMNOV V, WOGGON U, USTINOVICH E, ARTEMYEV M. Exciton-plasmon interaction in a composite metal-insulator-semiconductor nanowire system [J]. J Am Chem Soc, 2007, 129(48): 14939-14945.
[6] TRAN M L, CENTENO S P, HUTCHISON J A, ENGELKAMP H, LIANG D, TENDELOO G V, SELS B F, HOFKENS J, UJII H. Control of surface plasmon localization via self-assembly of silver nanoparticles along silver nanowires [J]. J Am Chem Soc, 2008, 130(51): 17240-17241.
[7] BAIK J M, LEE S J, MOSKOVITS M. Polarized surface-enhanced raman spectroscopy from molecules adsorbed in nano-gaps produced by electromigration in silver nanowires [J]. Nano Lett, 2009, 9(2): 672-676.
[8] LEE S J, MORRILL A R, MOSKOVITS M. Hot spots in silver nanowire bundles for surface-enhanced Raman spectroscopy [J]. J Am Chem Soc, 2006, 128(7): 2200-2201.
[9] FANG C, AGARWAL A, WIDJAJA E, GARLAND M V, WONG S M, LINN L, KHALID N M, SALIM S M, BALASUBRAMANIAN N. Metallization of silicon nanowires and sers response from a single metallized nanowire [J]. Chem Mater, 2009, 21(15): 3542-3548.
[10] CHRISTOPHER P, LINIC S. Engineering selectivity in heterogeneous catalysis: Ag nanowires as selective ethylene epoxidation catalysts [J]. J Am Chem Soc, 2008, 130(34): 11264-11265.
[11] CLAYTON D A, BENOIST D M, ZHU Y, PAN S L. Photoluminescence and spectroelectrochemistry of single Ag nanowires [J]. ACS Nano, 2010, 4(4): 2363-2373.
[12] HU L B, KIM H S, LEE J Y, PEUMANS P, CUI Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes [J]. ACS Nano, 2010, 4(5): 2955-2963.
[13] LU J J, YANG L B, XIE A J, SHEN Y H. DNA-templated photo-induced silver nanowires: Fabrication and use in detection of relative humidity [J]. Biophys Chem, 2009, 145(2-3): 91-97.
[14] STAWI?SKI G W, ZAMBORINI F P. Synthesis and alignment of silver nanorods and nanowires and the formation of Pt, Pd, and core/shell structures by galvanic exchange directly on surfaces [J]. Langmuir, 2007, 23(20): 10357-10365.
[15] HUNYADI S E, MURPHY C J. Bimetallic silver-gold nanowires: Fabrication and use in surface-enhanced Raman scattering [J]. J Mater Chem, 2006, 16(40): 3929-3935.
[16] BI Y P, LU G X. Facile Synthesis of platinum nanofiber/nanotube junction structures at room temperature [J]. Chem Mater, 2008, 20(4): 1224-1226.
[17] YANG R, SUI C H, GONG J, QU L Y. Silver nanowires prepared by modified AAO template method [J]. Mater Lett, 2007, 61(3): 900-903.
[18] HU Z A, KONG C, HAN Y X, ZHAO H X, YANG Y Y, WU H Y. Large-scale synthesis of defect-free silver nanowires by electrodeless deposition [J]. Mater Lett, 2007, 61(18): 3931-3934.
[19] DALCHIELE E A, MAROTTI R E, CORTES A, RIVEROS G, G?MEZ H, MART?NEZ L, ROMERO R, LEINEN D, MARTIN F, RAMOS-BARRADO J R. Silver nanowires electrodeposited into nanoporous templates: Study of the influence of sizes on crystallinity and structural properties [J]. Physica E, 2007, 37(1-2): 184-188.
[20] YU H B, PENG J, ZHAI M L, LI J Q, WEI G S. Silver nanowires formed within multi-walled carbon nanotubes by radiation-induced reduction of silver ions [J]. Physica E, 2008, 40(8): 2694-2697.
[21] CUI S Q, LIU Y C, YAN Z S, WEI X W. Construction of silver nanowires on DNA template by an electrochemical technique [J]. Mater Des, 2007, 28(2): 722-725.
[22] WEI G, ZHOU H L, LIU Z G, SONG Y H, WANG L, SUN L I, LI Z. One-step synthesis of silver nanoparticles, nanorods, and nanowires on the surface of DNA network [J]. J Phys Chem B, 2005, 109(18): 8738-8743.
[23] PARK S H, BARISH R, LI H Y, REIF J H, FINKELSTEIN G, YAN H, LABEAN T H. Three-helix bundle DNA tiles self-assemble into 2D lattice or 1D templates for silver nanowires [J]. Nano Lett, 2005, 5(4): 693-696.
[24] ZHANG D B, QI L M, MA J M, CHENG H M. Formation of silver nanowires in aqueous solutions of a double-hydrophilic block copolymer [J]. Chem Mater, 2001, 13(9): 2753-2755.
[25] ZHANG D B, QI L L, YANG J H, MA J M, CHENG H M, HUANG L. Wet chemical synthesis of silver nanowire thin films at ambient temperature [J]. Chem Mater, 2004, 16(5): 872-876.
[26] BAI J W, QIN Y, JIANG C Y, QI L L. Polymer-controlled synthesis of silver nanobelts and hierarchical nanocolumns [J]. Chem Mater, 2007, 19(14): 3367-3369.
[27] PENG C Y, LIU Z L, LI Z. Aggregation-based growth of silver nanowires at room temperature [J]. Appl Surf Sci, 2008, 254(9): 2581-2587.
[28] SUN Y H, GATES B, MAYERS B, XIA Y N. Crystalline silver nanowires by soft solution processing [J]. Nano Lett, 2002, 2(2): 165-168.
[29] WILEY B, SUN Y G, XIA Y N. Polyol synthesis of silver nanostructures: Control of product morphology with Fe(II) or Fe(III) species [J]. Langmuir, 2005, 21(18): 8077-8080.
[30] GAO Y, JIANG P, LIU D F, YUAN H J, YAN X Q, ZHOU Z P, WANG J X, SONG L, LIU L F, ZHOU W Y, WANG G, WANG C Y, XIE S S, ZHANG J M, SHEN D Y. Evidence for the monolayer assembly of poly(vinylpyrrolidone) on the surfaces of silver nanowires [J]. J Phys Chem B, 2004, 108(34): 12877-12881.
[31] CHEN D P, QIAO X L, QIU X L, CHEN J G, JIANG R Z. Convenient synthesis of silver nanowires with adjustable diameters via a solvothermal method [J]. J Colloid Interface Sci, 2010, 344(2): 286-291.
[32] LUU Q N, DOORN J M, BERRY M T, JIANG C Y, LIN C K, MAY P S. Preparation and optical properties of silver nanowires and silver-nanowire thin films [J]. J Colloid Interface Sci, 2011, 356(1): 151-158.
[33] DU J M, HAN B X, LIU Z M, LIU Y Q, KANG D J. Control synthesis of silver nanosheets, chainlike sheets, and microwires via a simple solvent thermal method [J]. Cryst Growth Des, 2007, 7(5): 900-904.
[34] CASWELL K K, BENDER C M, MURPHY C J. Seedless, surfactantless wet chemical synthesis of silver nanowires [J]. Nano Lett, 2003, 3(5): 667-669.
[35] YANG Z Q, QIAN H J, CHEN H Y, ANKER J N. One-pot hydrothermal synthesis of silver nanowires via citrate reduction [J]. J Colloid Interface Sci, 2010, 352(2): 285-291.
[36] BECKER R, S?DERLIND F, LIEDBERG B, K?LL P. Synthesis of silver nanowires in aqueous solutions [J]. Mater Lett, 2010, 64(8): 956-958.
[37] GU X, NIE C G, LAI Y K, LIN C J. Synthesis of silver nanorods and nanowires by tartrate-reduced route in aqueous solutions [J]. Mater Chem Phys, 2006, 96(2-3): 217-222.
[38] SARKAR R, KUMBHAKAR P, MITRA A K, GANEEV R A. Synthesis and photoluminescence properties of silver nanowires [J]. Curr Appl Phys, 2010, 10(3): 853-857.
[39] HE X, ZHAO X J, CHEN Y X, FENG J Y, SUN Z Y. Synthesis and characterization of silver nanowires with zigzag morphology in N,N-dimethylformamide [J]. J Solid State Chem, 2007, 180(8): 2262-2267.
[40] XU J, HU J, PENG C J, LIU H L, HU Y. A simple approach to the synthesis of silver nanowires by hydrothermal process in the presence of gemini surfactant [J]. J Colloid Interface Sci, 2006, 298(2): 689-693.
[41] MAILLARD M, HUANG P, BRUS L. Silver nanodisk growth by surface plasmon enhanced photoreduction of adsorbed [Ag+] [J]. Nano Lett, 2003, 3(11): 1611-1615.
[42] SUN Y G, MAYERS B, XIA Y N. Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process [J]. Nano Lett, 2003, 3(5): 675-679.
[43] HU J Q, CHEN Q, XIE Z X, HAN G B, WANG R H, REN B, ZHANG Y, YANG Z L, TIAN Z Q. A simple and effective route for the synthesis of crystallines silver nanorods and nanowires [J]. Adv Funct Mater, 2004, 14(2): 183-189.
[44] ZHANG W J, LIU Y, CAO R G, LI Z H, ZHANG Y H, TANG Y, FAN K N. Synergy between crystal strain and surface energy in morphological evolution of five-fold-twinned silver crystals [J]. J Am Chem Soc, 2008, 130(46): 15581-15588.
[45] ZHANG H, MIRKIN C A. DPN-generated nanostructures made of gold, silver, and palladium [J]. Chem Mater, 2004, 16(8): 1480-1484.
[46] HUANG F, ZHANG H Z, BANFIELD J F. Two-stage crystal-growth kinetics observed during hydrothermal coarsening of nanocrystalline ZnS [J]. Nano Lett, 2003, 3(3): 373-378.
一种新颖、简单的银微米线的湿化学制备方法
翟爱霞1,蔡雄辉2,姜晓晔2,范国枝1
1. 武汉工业学院 化学与环境工程学院,武汉 430023;
2. 武汉工业学院 生物与制药工程学院,武汉 430023
摘 要:提供一种新颖简单的银微米线的湿化学制备方法。在反应温度为50 ℃的条件下,把硫酸亚铁溶液逐渐滴加到含有柠檬酸的硝酸银溶液中,合成银微晶体。采用扫描电镜(SEM)和X射线衍射仪(XRD)对所制备的银微晶体进行表征。结果表明:所制备的银微晶体主要由大量的银微米线组成;银微晶体的形态与反应温度有很强的联系;当硝酸银浓度降低时,银微米线的长度和直径都逐渐增大,且较低的硝酸银浓度不利于生成更多的银微米线。降低硫酸亚铁浓度时也出现类似的结果。柠檬酸的用量对银微晶体的微观形态有很大的影响。根据银微米线的形成机理,推断柠檬酸在Oswald熟化形成银微米线的过程中起着非常重要的作用。
关键词:银微米线;硫酸亚铁;柠檬酸;湿化学法
(Edited by YANG Hua)
Foundation item: Project (2011CDC114) supported by the Hubei Provincial Natural Science Foundation of China
Corresponding author: ZHAI Ai-xia; Tel: +86-27-83943956; E-mail: zaisfte@163.com
DOI: 10.1016/S1003-6326(11)61268-5